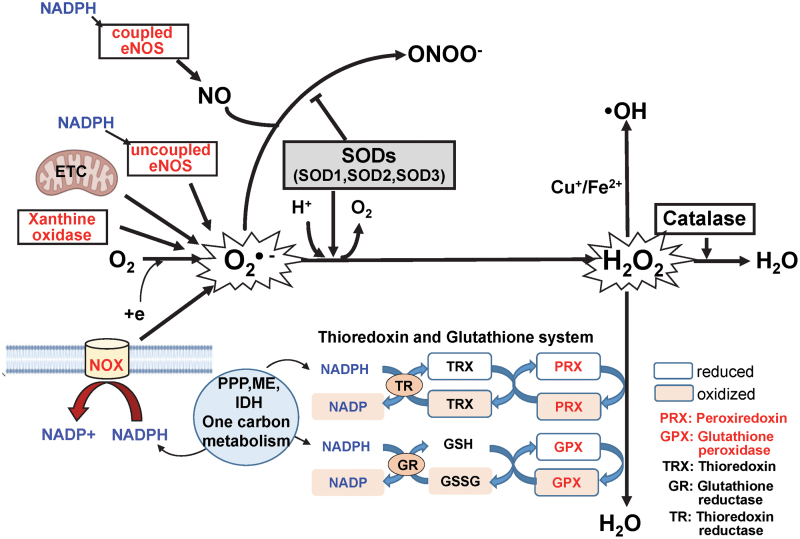
FIG. 2.
Generation and metabolism of ROS/RNS. O2•− is produced by NOXs, the mitochondrial ETC, XO, lipoxygenase, cyclooxygenase, and uncoupled NOS. O2•− is converted by SODs to H2O2, which, in turn, is reduced to water via the actions of catalase, GPXs, and PRXs. The PRX/TRX and GPX/GSH systems are fueled by NADPH, which is generated by the PPP, IDHs, MEs, and 1C metabolism. Of note, NADPH is also a substrate for the ROS-generating NOXs and NOS. In the presence of reduced transition metals (Fe2+ and Cu2+), H2O2 undergoes spontaneous conversion to reactive OH• or related metal-associated reactive species. NO• is produced by coupled NOS. The NOS enzymes utilize NADPH and l-arginine as co-substrates and BH4 (a product of 1C metabolism) as essential co-factors. Although all NOS isoforms generate NO•, they can also generate O2•− at the expense of NO• via a process known as uncoupling. The mechanisms underlying the uncoupling process include the formation of monomers, altered Hsp90 binding, and insufficient levels of BH4 and l-arginine. Importantly, NO• can be rapidly inactivated via a reaction with O2•−, which leads to the formation of the strong oxidant, ONOO−. Thus, SODs are the first line of defense against O2•−-mediated toxicity. The SODs also participate in cell signaling events via their capacity to regulate levels of ROS (e.g., O2•−, H2O2) while preserving available NO•. BH4, tetrahydrobiopterin; GPX, glutathione peroxidase; H2O2, hydrogen peroxide; Hsp, heat-shock protein; NO•, nitric oxide; NOS, nitric oxide synthase; NOX, NADPH oxidase; O2•−, superoxide; OH•, hydroxyl radical; ONOO−, peroxynitrite; PRX, peroxiredoxin; RNS, reactive nitrogen species; SOD, superoxide dismutase; XO, xanthine oxidase. Color images are available online.