FIG. 6.
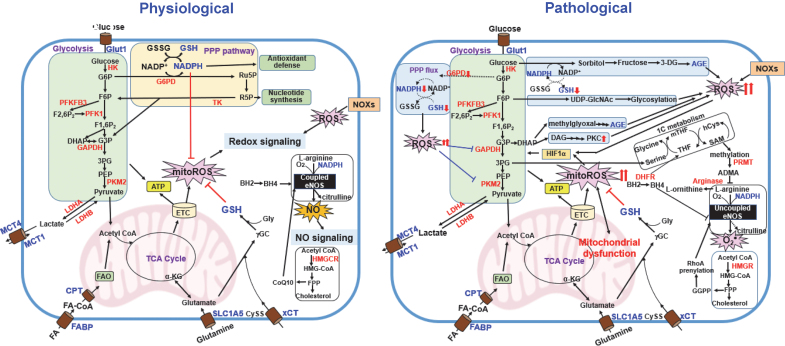
The interplay between metabolic pathways and ROS (redox homeostasis) in physiological and pathological states.Left panel, glycolysis plays an essential role in the control of redox homeostasis by generating PPP-derived NADPH that is involved in the antioxidant system. FAO also generates NADPH through metabolic reactions. Glutaminolysis is involved in redox control by increasing GSH synthesis. During mitochondrial metabolism, ROS will be produced via ETC. NO• is produced by NOS by utilizing BH4, NADPH, and molecular O2 to convert l-arginine to l-citrulline (coupled eNOS). NOXs- and mitochondria-derived ROS activate redox signaling. Right panel, in pathological states such as diabetes and atherosclerosis, excess ROS due to eNOS uncoupling and PPP-GSH impairment inhibit glycolytic flux, which diverts glycolytic intermediates into alternative metabolic pathways such as polyol pathway (AGE production) and PKC activation. This, in turn, further increases mitochondria- or NOX-derived ROS production and mitochondrial dysfunction. mitoROS or Nox-derived ROS stabilize HIF-1α, which induces a metabolic shift toward glycolysis, resulting in reduced oxidative phosphorylation and mitoROS production. The ADMA produced by l-arginine methylation via the 1C metabolism as well as arginase inhibit l-arginine binding to NOS, which decreases NO production. The mevalonate pathway also facilitates eNOS uncoupling by RhoAprenylation induced by GGPP, thereby promoting excess ROS production. ADMA, asymmetric dimethylarginine; GGPP, geranylgeranyl pyrophosphate; PKC, protein kinase C. Color images are available online.