Figure 1. ch128.1/IgG1 treatment of NSG mice implanted with EBV-exposed human B-cells leads to significantly reduced mortality and inhibits tumor development.
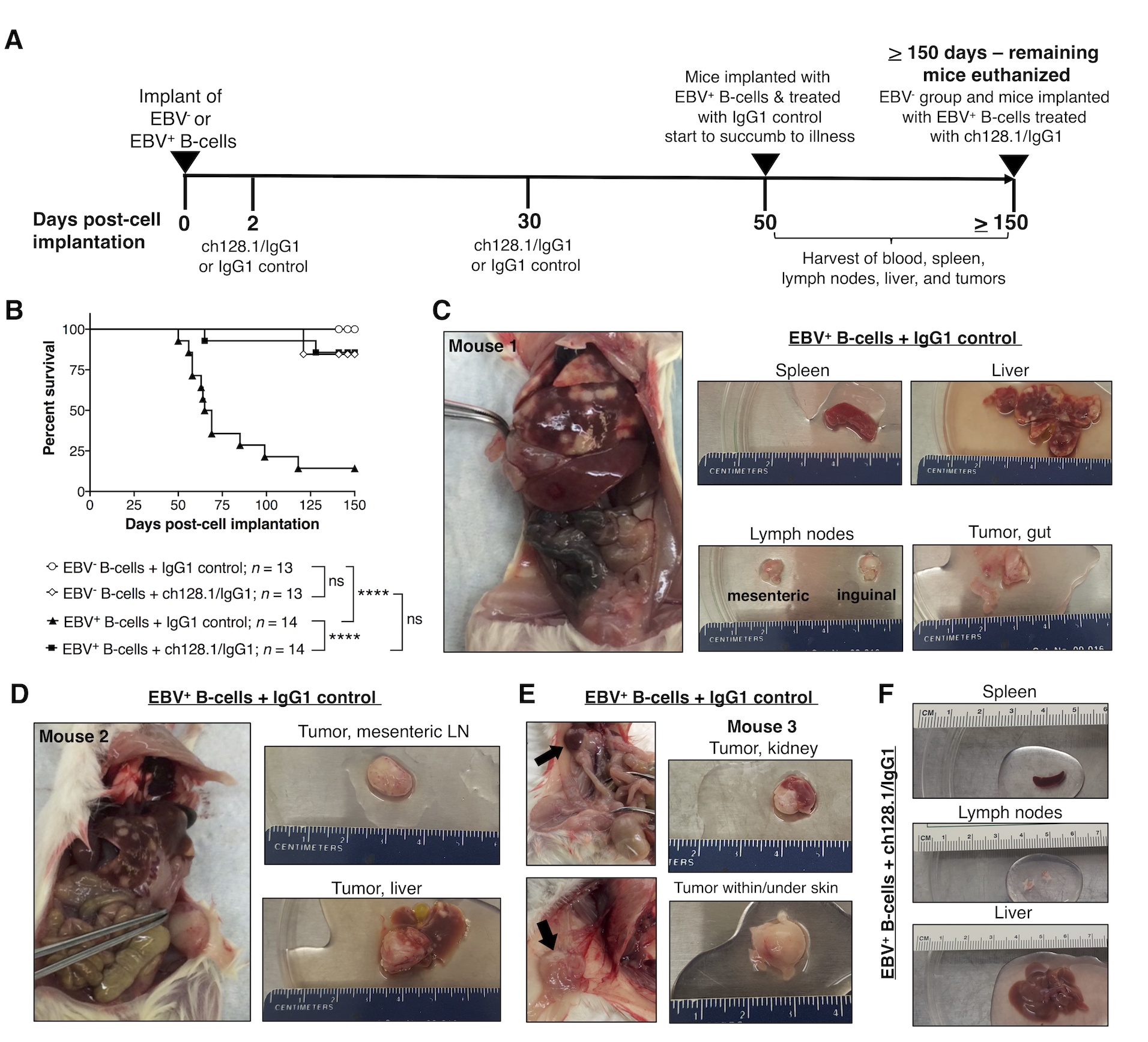
A, Experimental timeline of NSG mice implanted i.v. with 6 × 106 EBV− or EBV+ B-cell enriched preparations (T-cell depleted PBMCs). Two groups of mice received EBV− cells and two groups received EBV+ (cultured in vitro for 7 days with EBV prior to implantation). Mice were then treated with ch128.1/IgG1 (400 μg/mouse) or IgG1 control (400 μg/mouse) antibody at 2 days and 30 days post-cell implantation. Mice implanted with EBV+ cells and treated with IgG1 control started showing signs of disease and/or died after 50 days post-cell implantation, at which point, blood collected from cardiac puncture, spleen, lymph nodes, liver, and tissues with tumors were harvested. All surviving mice were euthanized at or after 150 days post-cell implantation. B, Kaplan-Meier survival curve for mice implanted with EBV− or EBV+ B-cells and treated with ch128.1/IgG1 or IgG1 control. Combined results are from 2 independent experiments. Statistical comparisons were conducted using the Mantel-Cox log-rank test, where **** indicates statistically significant difference (p < 0.0001) and ns, not significant. C, D, E, Necropsy and gross tissue images of mice that developed tumors [C, D, Mouse #1 and #2, respectively, at 58 days post-cell implantation; and E, Mouse #3, 65 days post-cell implantation]. F, Normal gross tissue images from a mouse implanted with EBV-exposed B-cells and treated with ch128.1/IgG1 antibody at 155 days post-cell implantation.
