Figure 3. NSG mice implanted with EBV+ B-cells and treated with IgG1 control antibody developed lymphoproliferative growths that were of human CD19+ B-cell origin and EBV-positive.
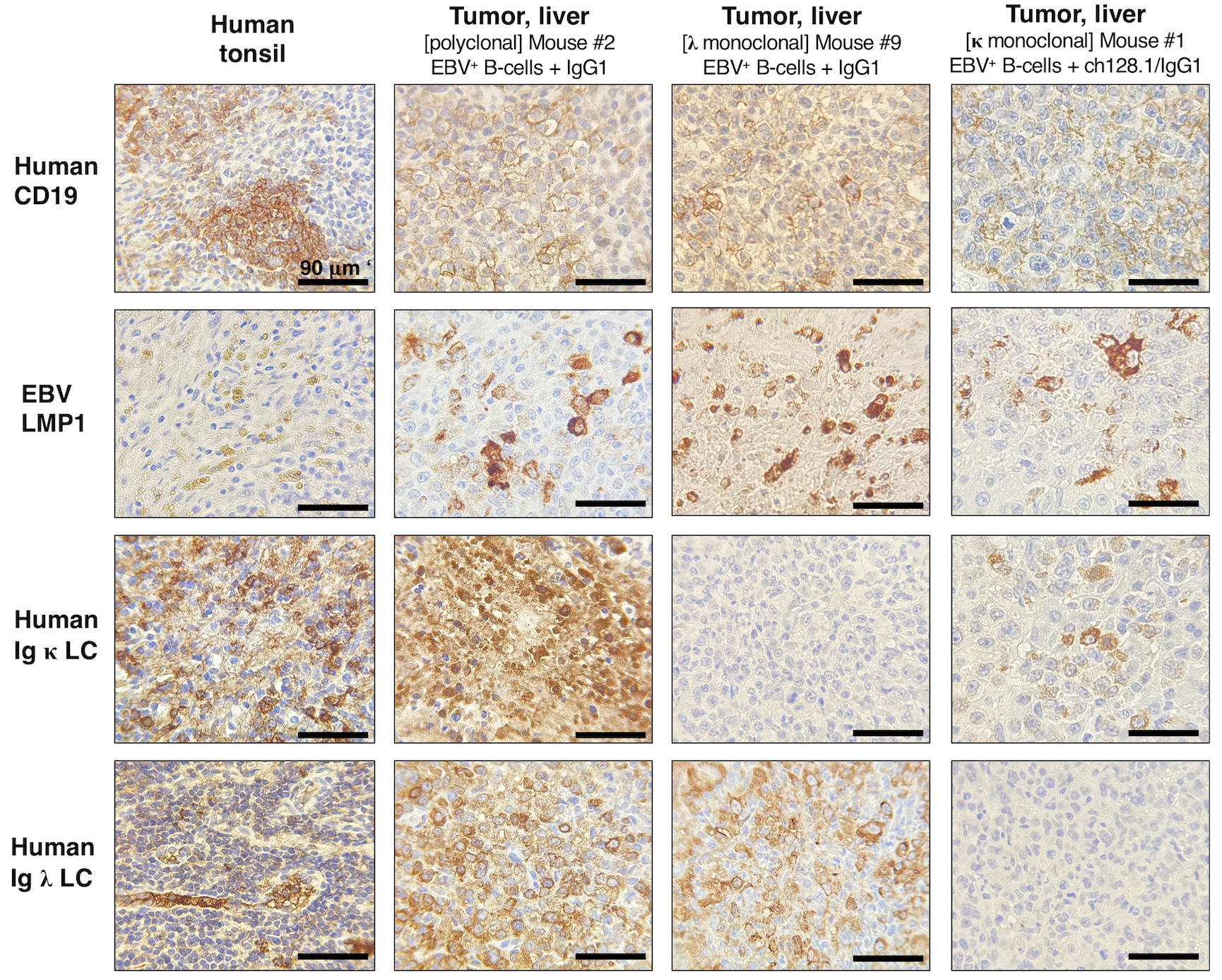
Left panels, Human tonsil tissue showing positive staining for human CD19+ B-cells, human Ig κ or Ig λ LC, and EBV infection (EBV LMP1+). Middle panels, Representative IHC staining in liver tissue sections from mice implanted with EBV+ B-cell enriched preparations and treated with IgG1 control monoclonal antibody. This mouse had human CD19+ and EBV+ polyclonal tumor growths (derived from different EBV+ B-cell clones) in liver tissue (middle panels), and mesenteric lymph nodes (images of Mouse #2 are shown in Fig. 1D and IHC results are summarized in Table 1). Right panels, IHC staining in liver tissue sections from a mouse implanted with EBV+ B-cells and treated with IgG1 control. This mouse had tumor growths in liver (shown) and splenic tissue sections (not shown) that were of human CD19+ B-cell origin and EBV LMP1+, as summarized in Table 1. Liver cells were human Ig κ LC negative and Ig λ LC positive, suggesting that these were monoclonal lymphoproliferative growths.
