Figure 6. Glutathione synthesis is directly regulated by RB loss-induced E2F1 function and is a candidate for therapeutic intervention.
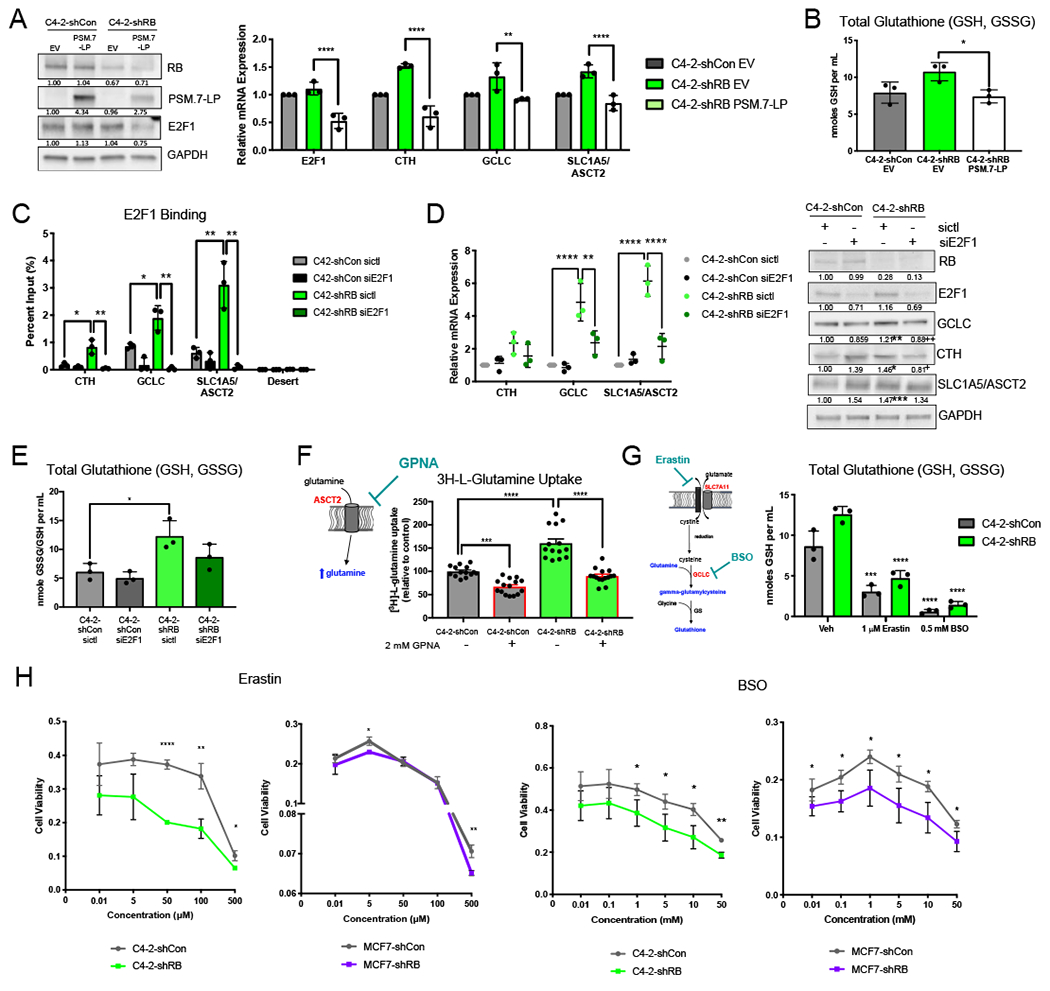
(A) Protein expression of RB, PSM.7-LP, and E2F1 after transfection of 1 μg of PSM.7-LP (left). mRNA expression of E2F1 and glutathione synthesis genes after RB loss and rescue with PSM.7-LP (right). (B) Total glutathione after RB knockdown and rescue with PSM.7-LP. (C) Validation of E2F1 binding at glutathione synthesis gene promoters after RB knockdown. E2F1 binding is lost following transient knockdown of E2F1. (D) mRNA expression of targets after RB knockdown and treatment with siControl or siE2F1 (left). Validation of protein expression of glutathione metabolism enzymes after RB1 depletion with treatment of siControl or siE2F1 (right). Blot quantification is shown below bands. (E) Total glutathione with and without RB knockdown after treatment with siControl or siE2F1. (F) Glutamine uptake assay showing increased glutamine uptake after RB knockdown. C4-2-shCon and C4-2-shRB were treated with 2 mM of the SLC1A5/ASCT2 transporter inhibitor, L-γ-Glutamyl-p-nitroanilide (GPNA). (G) Total glutathione following treatment with either SLC7A11 inhibition via Erastin or GCLC inhibition via BSO, in control and RB knockdown models. (H) Cytotoxicity of varying concentrations of Erastin and BSO in control and RB knockdown CRPC and BrCa models *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001.
