FIGURE 1.
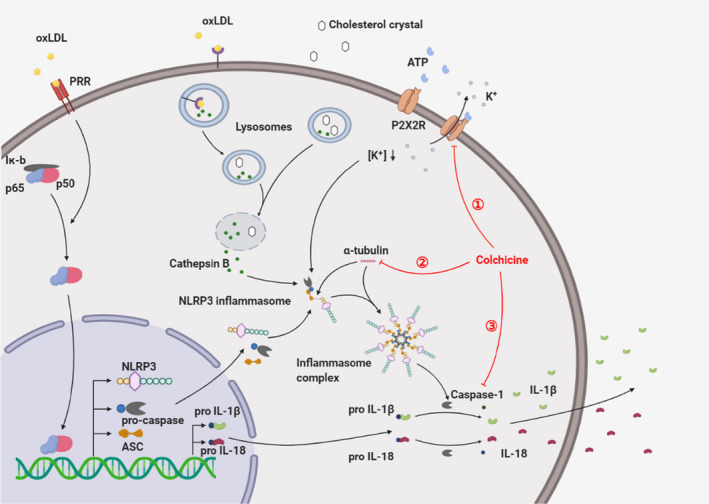
Inflammasome assembly and activation mechanism. OxLDL can activate the NF‐κB signal transduction pathway through PRR, which not only increases the expression of NLRP3, pro‐Caspase and ASC, but also upregulates the pro‐IL‐1β and pro‐IL‐18 levels. Extracellular ATP can combine with P2X2R to activate P2X2R, increase K+ efflux and decrease intracellular K+ concentration, which provides a basis for the assembly and activation of NLRP3 inflammasomes. At the same time, OxLDL can be swallowed by macrophages through membrane receptors and can be converted into cholesterol crystals in the lysosome. The CCs (formed intracellularly or derived extracellularly) can cause the lysosome to rupture, resulting in Cathepsin B being released into the cytoplasm and inflammasome activation. Microtubules can transport ASC so that it can combine with NLRP3 and assemble NLRP3 inflammasomes into complexes. After the inflammasome is activated, pro‐Caspase can be converted into Caspase by shearing, and the activated Caspase can cleave pro‐IL‐1β and pro‐IL‐18 into IL‐1β and IL‐18, and secrete them outside the cell, causing an outbreak of inflammation. However, colchicine can inhibit the activation of NLRP3 inflammasomes and reduce the release of IL‐1β through a variety of ways to inhibit inflammation, mainly in three ways as follows: ① restriction of P2X7 receptor and reduction of K+ outflow; ② damping of microtubule synthesis, and inhibition of the assembly of NLRP3 inflammasome and NLRP3 inflammasome complex; ③ inhibition of NLRP3 inflammasome activation and IL‐1 β release
