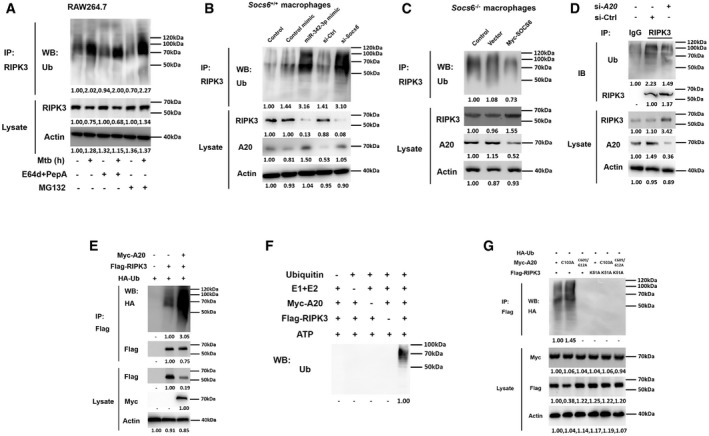
Figure 7. A20 controls degradation of RIPK3 through ubiquitin chains.
-
ARAW264.7 cells were pretreated with or without E64d/PepA or MG132 for 4 h and then stimulated with or without Mtb for 12 h as indicated. Cell lysates were collected and immunoprecipitated using an anti‐RIPK3 antibody, and the ubiquitination of endogenous RIPK3 was detected using an anti‐Ub antibody. Representative blots from n = 3 biological replicates are shown.
-
B, CSocs6+/+ BMDMs were transfected with miR‐342‐3p mimic or Socs6 siRNA (B), and Socs6 −/− BMDMs were transfected with plasmid expressing Myc‐SOCS6 (C) for 24 h as indicated. Transfected cells were then stimulated with Mtb for another 12 h, cell lysates were collected for immunoprecipitation, and ubiquitination of endogenous RIPK3 was detected by immunoblot. Representative blots from n = 3 biological replicates are shown.
-
DRAW264.7 cells were transfected with A20 siRNA, and cell lysates were collected for immunoprecipitation and immunoblot. Representative blots from n = 3 biological replicates are shown.
-
E–GRAW264.7 cells were transfected with plasmids expressing A20 and RIPK3 (E), or A20 and RIPK3 mutants (G) as indicated, cell lysates were collected for immunoprecipitation and immunoblot. Myc‐A20 or Flag‐RIPK3 purified from transfected HEK‐293T cells was incubated with ATP, E1, E2, and ubiquitin as indicated. The in␣vitro ubiquitination of RIPK3 was analyzed by immunoblot using an anti‐Ub antibody (F). Representative blots from n = 3 biological replicates are shown.
Source data are available online for this figure.
