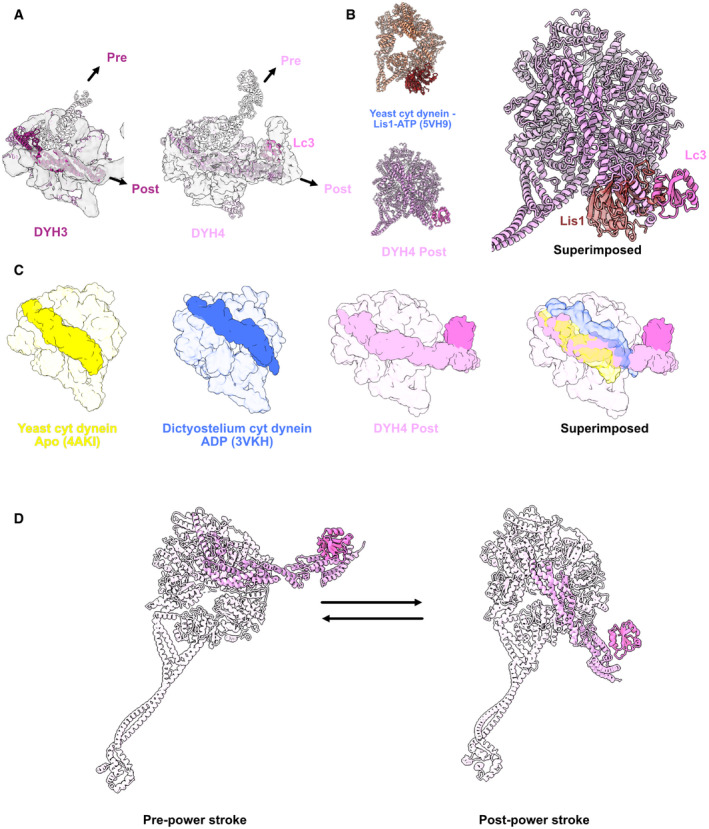
Figure 4. Structure of the head domain in ODA on the doublet.
-
AModels of the Dyh3 and Dyh4 heads in post‐powerstroke conformations are fitted in the cryo‐EM maps of the ODA on the doublet. The Dyh3 and Dyh4 linkers in pre‐powerstroke conformations in the inactive Shulin–ODA model are also superimposed (shown in transparent).
-
BComparison of yeast cytoplasmic dynein bound to Lis1 (PDB: 5VH9) with the Dyh4 bound to LC3. LC3 also binds to the AAA+ ring but in an opposite site from Lis1.
-
CComparison of the linker of Dyh4 in post‐powerstroke conformation with the yeast cytoplasmic dynein in apostate and dictyostelium cytoplasmic dynein in ADP state reveals that the Dyh4 linker is positioned in between of the linker positions in apo and ADP states. The structures are aligned based on the AAA+ ring without linker.
-
DModel for the conformation changes from pre‐powerstroke to post‐powerstrokes in Dyh4.
