Figure 5.
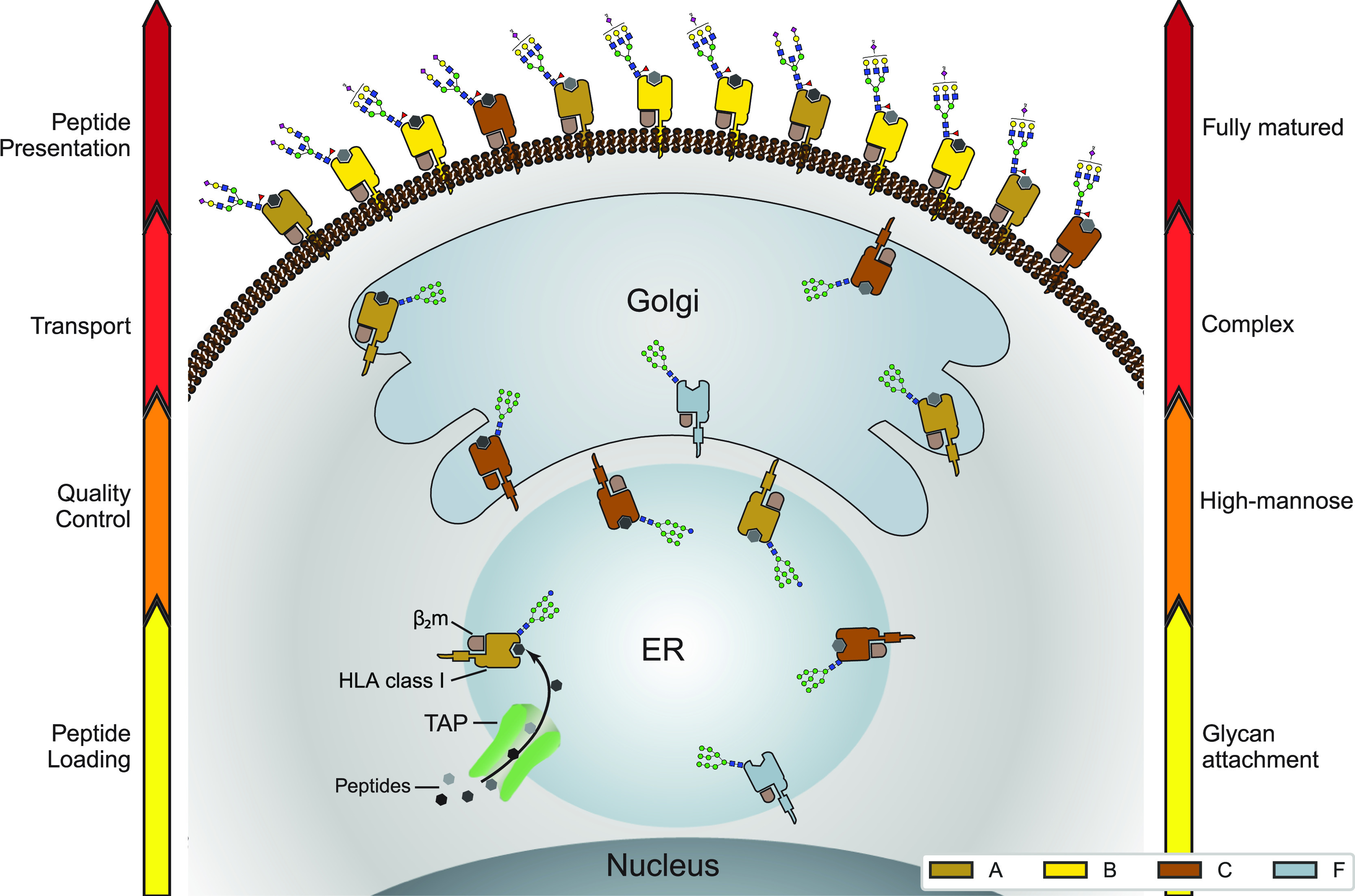
Distinctive glycosylation and cellular localization of HLA class I proteins. Starting as nascent chains synthesized by cellular ribosomes, HLA heavy chains traverse through specific IM compartments to get properly folded, trimmed, associated with the β2m chain, loaded with the peptide antigens, and glycosylated. These processes occur largely sequential and require chaperones, the PLC and a variety of glycoenzymes, residing in the different subcompartments of the ER and Golgi. Fully assembled, peptide-loaded, and maturely glycosylated HLA complexes make it to the cell surface becoming embedded in the PM, where they present the peptides to the T-cell receptors of CD8+ T-cells. The arrows on either side of the schematic indicate the stages of HLA antigen expression (left) and glycosylation maturation (right). The HLA complexes of different class I genes are color coded corresponding to the colors used in Figures 2A and 4A. The number of copies presented illustrates roughly their relative distribution over the IM compartments and PM in JY cells. Moreover, prototypical HLA glycans observed uniquely in the IM fraction and PM fractions are depicted as well.
