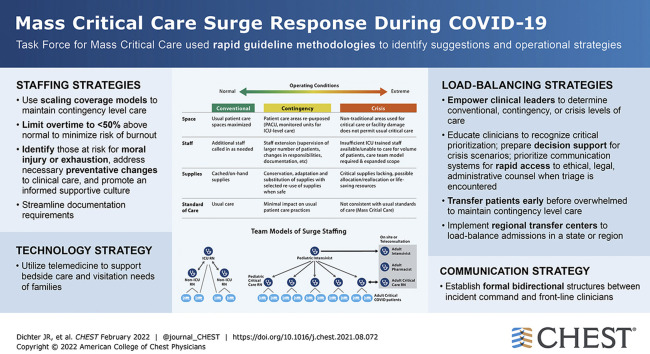
Abstract
Background
After the publication of a 2014 consensus statement regarding mass critical care during public health emergencies, much has been learned about surge responses and the care of overwhelming numbers of patients during the COVID-19 pandemic. Gaps in prior pandemic planning were identified and require modification in the midst of severe ongoing surges throughout the world.
Research Question
A subcommittee from The Task Force for Mass Critical Care (TFMCC) investigated the most recent COVID-19 publications coupled with TFMCC members anecdotal experience in order to formulate operational strategies to optimize contingency level care, and prevent crisis care circumstances associated with increased mortality.
Study Design and Methods
TFMCC adopted a modified version of established rapid guideline methodologies from the World Health Organization and the Guidelines International Network-McMaster Guideline Development Checklist. With a consensus development process incorporating expert opinion to define important questions and extract evidence, the TFMCC developed relevant pandemic surge suggestions in a structured manner, incorporating peer-reviewed literature, “gray” evidence from lay media sources, and anecdotal experiential evidence.
Results
Ten suggestions were identified regarding staffing, load-balancing, communication, and technology. Staffing models are suggested with resilience strategies to support critical care staff. ICU surge strategies and strain indicators are suggested to enhance ICU prioritization tactics to maintain contingency level care and to avoid crisis triage, with early transfer strategies to further load-balance care. We suggest that intensivists and hospitalists be engaged with the incident command structure to ensure two-way communication, situational awareness, and the use of technology to support critical care delivery and families of patients in ICUs.
Interpretation
A subcommittee from the TFMCC offers interim evidence-informed operational strategies to assist hospitals and communities to plan for and respond to surge capacity demands resulting from COVID-19.
Key Words: contingency, conventional, COVID-19, crisis levels, critical clinical prioritization, incident command system, load-balancing, mass critical care, staffing, surge, telemedicine, tiered staffing
Abbreviations: CCP, critical clinical prioritization; HCW, health care worker; PD, peritoneal dialysis; TFMCC, Task Force for Mass Critical Care
Graphical Abstract
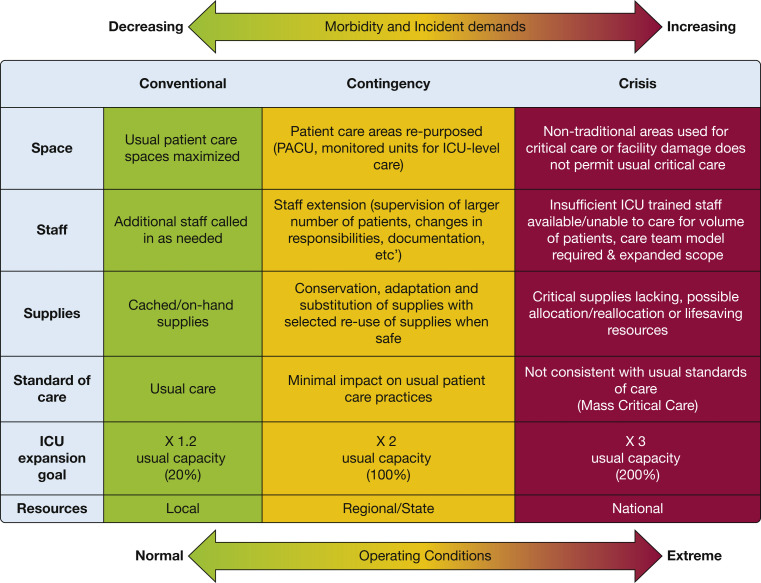
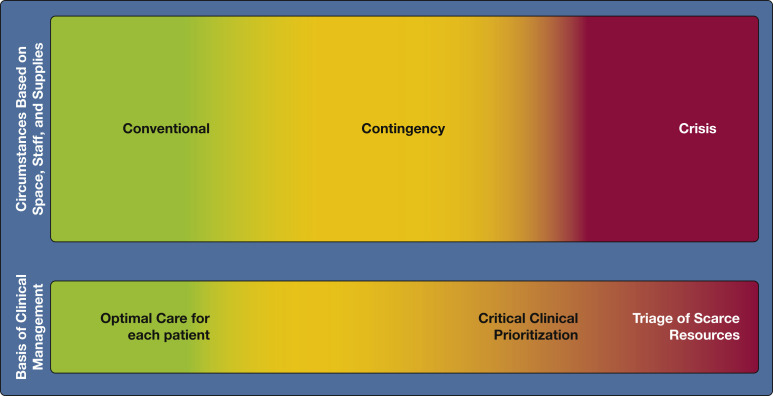
The COVID-19 pandemic confronted hospitals with unprecedented surges of seriously ill patients.1 , 2 In 2014, the Task Force for Mass Critical Care (TFMCC) developed consensus statement suggestions for the provision of care during pandemics in collaboration with the American College of Chest Physicians.3 The concepts of increasing critical care bed numbers and augmenting staff, equipment, supplies, and describe operational strategies to scale up surge staffing effectively and maintain contingency-level medications (“space, stuff, staff”) provided an effective framework for hospitals confronted by COVID-19 (Fig 1 ).4 , 5 It is disappointing that these were not operationalized to the degree necessary to prevent adverse outcomes.6 , 7
Figure 1.
Diagram showing a framework for critical care surge capacity planning outlining the conventional, contingency, and crisis surge responses. PACU = post-anesthesia care unit. (Reprinted with permission from Christian et al.3)
As foreseen, supply chain disruptions led to shortages of key medications, consumables, and personal protective equipment.8 , 9 Hospital space became a premium as critically ill patients overflowed from full ICUs into post-anesthesia care units, EDs, operating rooms, intermediate and monitored units, flat-space areas, and even temporary or tent facilities, with up to 25% of COVID-19 deaths attributable to increased hospital surge caseload.1 , 10 The prolonged course of COVID-19 has led to contingency conditions becoming the norm for months. More than 3,600 US health care workers (HCWs) have died.11
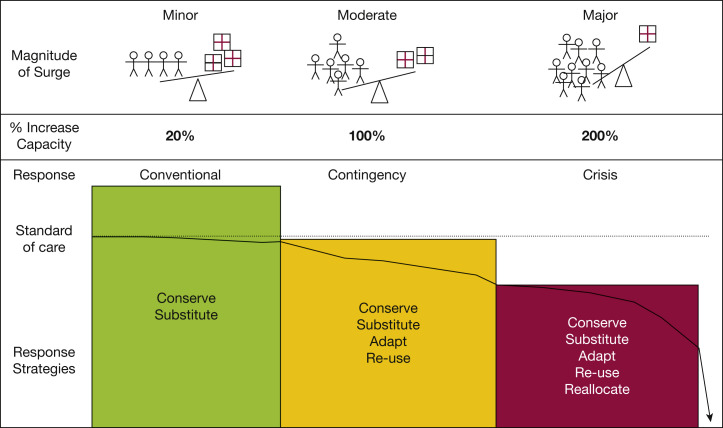
Contingency responses refer to increasing hospital resources by repurposing equipment and supplies, augmenting the clinical workforce, and expanding care to nontraditional areas of the hospital while maintaining functionally equivalent standards of care.4 , 5 Figure 2 demonstrates the transition from contingency to crisis where contingency level care is not sustainable and care is prioritized or limited, leading to substantial risk of adverse outcomes and potential triage of scarce resources.3 , 12 Notably, no so-called bright line exists between risk levels that separates contingency from crisis, and it is imperative that neighboring hospitals adopt similar strategies.
Figure 2.
Diagram depicting the spectrum of surge from minor through major. The magnitude of surge is illustrated by the alterations in the balance between demand (stick figures) and supply (medication boxes). As surge increases, the demand-supply imbalance worsens. Conventional, contingency, and crisis responses are used to respond to the varying magnitude of surge. Varying response strategies are associated with each level of response. As the magnitude of the surge increases, the strategies used to cope with the response gradually depart from the usual standard of care (default defining the standards of disaster care) until such point that even with crisis care, critical care is no longer able to be provided. (Reprinted with permission from Christian et al.3)
Ten new suggestions are presented in this interim report emphasizing specific operational strategies intended to prolong the contingency state, thereby avoiding crisis and the need for triage of scarce resources. These suggestions are based upon data and experiences from COVID-19 surge mitigation that, when implemented, will maintain contingency-level care and will prevent or delay transition to crisis.
Methods
The TFMCC is composed of a interdisciplinary group of disaster professionals including physicians, nurses, pharmacists, respiratory therapists, and health system leaders experienced in the management of critically ill patients with COVID-19. The TFMCC adopted a modified version of established rapid guidelines from the World Health Organization13 and Guidelines International Network-McMaster Guideline Development Checklist14 with our previous methodology.15 Subcommittee members voted on initial suggestions using a five-point Likert scale, derived from the Grading of Recommendations Assessment, Development and Evaluation grid.16 , 17 Three distinct areas of surge preparedness and management related to the COVID-19 pandemic were prioritized and include (1) communication and coordination, (2) staffing and resilience, and (3) communications and technology. See e-Appendix 1 for complete methodology.
Data Extraction
The TFMCC members believe it is urgent to distribute the most important and relevant information widely based on preliminary work, generating this interim communication. Each subcommittee conducted a literature review of published evidence relevant to their respective area. Studies published since 2020 were prioritized for inclusion due to direct evidence addressing COVID-19. Key narrative statements deemed relevant were extracted and arranged into overarching themes. The TFMCC members also met weekly to share anecdotal evidence from their own experiences managing COVID-19 surge. The statements extracted from the literature review were combined with the anecdotal evidence for each theme, when applicable, to arrive at initial suggestions for each subcommittee.
Results
The TFMCC members identified four issues requiring urgent attention to address future surges including staffing, load-balancing, communications, and technology, resulting in 10 suggestions with corresponding operational strategies (Table 1 ).
Table 1.
Summary of 10 Suggestions and Operational Strategies
| Suggestions | Operational Strategy | Category |
|---|---|---|
| 1. We suggest graded staff-to-patient ratios with consideration to experience level, resources, and patient acuity to optimize contingency care and avoid crisis care (Figs 3, 4, 5). | Three staffing models are presented to scale up surge staffing effectively to maintain contingency-level care. | Staffing |
| 2. We suggest limiting overtime to less than 50% above normal for all HCWs to minimize the risk of burn-out and exhaustion. | Limit overtime to < 50% more than normal for all staff to minimize risk of burnout. | Staffing |
| 3. We suggest that the mental health needs of all HCWs are priorities for maintaining an effective response and staffing capacity. | Identify HCWs at risk of moral injury or exhaustion, address necessary preventative changes to clinical care, and promote an informed supportive culture. | Staffing |
| 4. During surge, we suggest minimizing redundant clinical documentation requirements to focus on core elements directly relevant to bedside care. | Responsibly streamline documentation requirements. | Staffing |
| 5. We suggest that resource strain level be actively monitored and determined by front line clinical leaders based upon assessment of available resources and conditions. | Clinical leaders, ICU directors, and service chiefs should be empowered to determine local resources including strain indicators as being conventional, contingency, or at crisis levels. | Load-balancing |
| 6. We suggest there is a transition zone toward the limits of contingency care when increasingly scarce resources are modified beyond routine standards of care to preserve life. This critical clinical prioritization level precedes triage of scarce resources and is a powerful indicator for needed resources to maintain contingency-level care. (case study Fig 8) | Educate clinicians to recognize critical clinical prioritization to request resources or patient transfers; prepare decision support for potential crisis scenarios; prioritize communication systems for rapid access to ethical, legal, and administrative counsel when triage of scarce resources is encountered. | Load-balancing |
| 7. We suggest that early transfer of patients before a hospital is overwhelmed promotes the effective conservation of resources and less deviation from routine care standards. | Transfer (load-balance) patients early before a hospital is overwhelmed to maintain contingency-level care. | Load-balancing |
| 8. We suggest earlier utilization of regional transfer centers for load-balancing during surge for patient transfers and placement. We also suggest having intensivist or hospitalist availability to help prioritize transfers and provide support to bedside clinicians when transfers are delayed. | Implement regional transfer centers to improve bed access and assure efficient ICU bed use through active management and load-balancing of admissions across all hospitals in a state or region. On-call intensivist or hospitalist support should be available as a resource. | Load-balancing |
| 9. We re-emphasize that designated clinicians who are actively engaged in clinical work (especially intensivists and hospitalists) actively participate in hospital incident command structure; this group should provide updates to clinical staff for improving situational awareness, ensuring bidirectional communication. | Establish formal communication structures between incident command and frontline clinicians, such as PCSS or PCCS team to ensure bidirectional communication and situational awareness. | Communication |
| 10. We suggest hospitals apply telemedicine technology to augment critical care early and in the broadest sense possible. | Use telemedicine technology to support bedside critical care, to connect specialty clinicians to distant sites, and to support visitation needs of families. | Technology |
HCW = health care worker; PCSS = physician clinical support supervisor.
Staffing Suggestions
During the COVID-19 pandemic, patients have died from the lack of staffed ICU beds.18, 19, 20 Rapid bed expansion must balance staff safety and quality of care, impact on providers, and impact outside of ICUs.21 The risks of repurposing and augmenting staff include (1) lower-quality care without adequate training, (2) excessive duty times and workload, (3) moral injury, (4) the costs of training new staff, and (5) the costs of cancelling time-sensitive but nonemergent care with associated potential adverse consequences.
Suggestion 1: We suggest graded staff-to- patient ratios with consideration to experience level, resources, and patient acuity to optimize contingency care and avoid crisis care ( Figs 3 , 4 , 5 ).
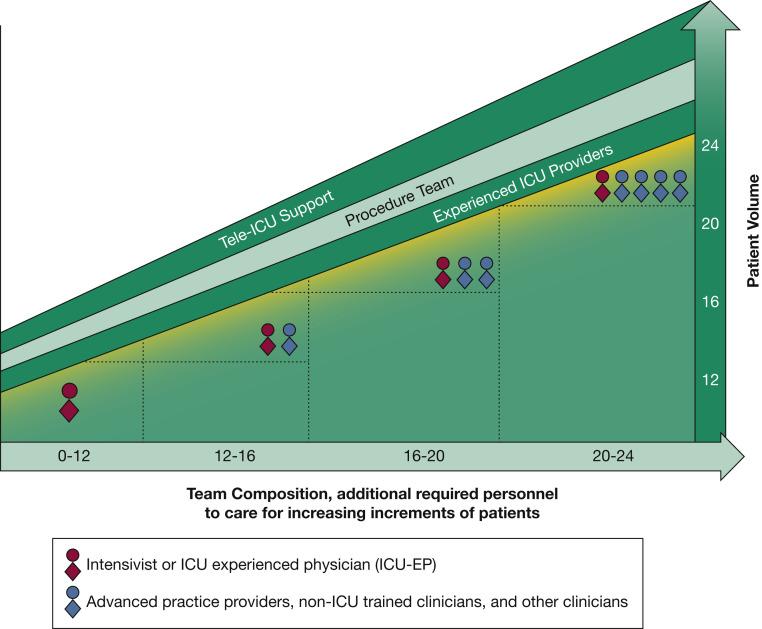
Figure 3.
Diagram showing critical care adult physician or provider staffing model for expanding surge coverage.22, 23, 24, 25, 26, 27, 28, 29 This model assumes all ICU care teams have at least one intensivist or other skilled ICU physician (red circle-and-diamond figures) to surge while maintaining contingency level care. An intensivist or skilled ICU physician may manage up to 12 patients in a 12-h shift when providing only direct hands-on care, and up to 24 patients when combining hands-on care with support and collaboration for up to four other clinician team members (blue circle-and-diamond figures). The factors that effectively increase an ICU team’s capacity to expand coverage while maintaining contingency level care include (banners at the top of graph): (1) team members with more ICU experience, (2) the presence of procedure teams (for placing invasive lines or other procedures, for intubations, for other care such as prone positioning), and (3) the presence of telemedicine support. Higher acuity patients (as is typical with patients with COVID-19 in the ICU) may impact capacity negatively by demanding greater resources to maintain contingency-level care. Other clinician team members may include non-ICU skilled physicians and ICU or non-ICU skilled practitioners, or both; training institutions trainees (residents and fellows) also may be team members. Finally, this model is focused on resources for direct patient care only, and sufficient resource needs for overnight staffing and cross-coverage also should be factored and scaled up appropriately with increasing surge.
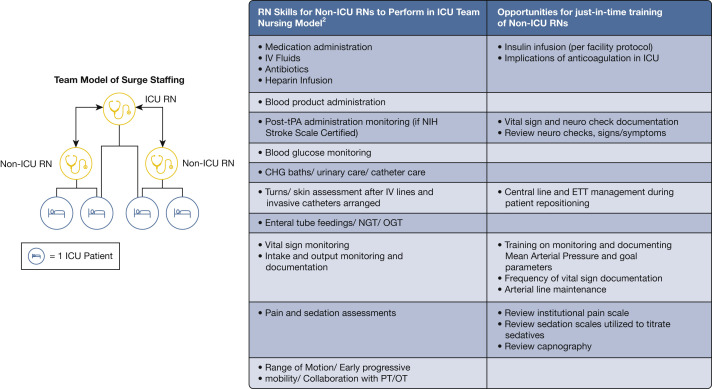
Figure 4.
Diagram showing critical care surge capacity team nursing staffing model.30,31 One ICU-trained RN working with two non-ICU-trained RNs can expand ICU level care to four patients by having each focus using their own skill sets. CHG = change; ETT = endotracheal tube; NGT = nasogastric tube; OGT = orogastric tube; OT = occupational therapy; PT = physical therapy; RN = registered nurse; tPA = tissue plasminogen activator.
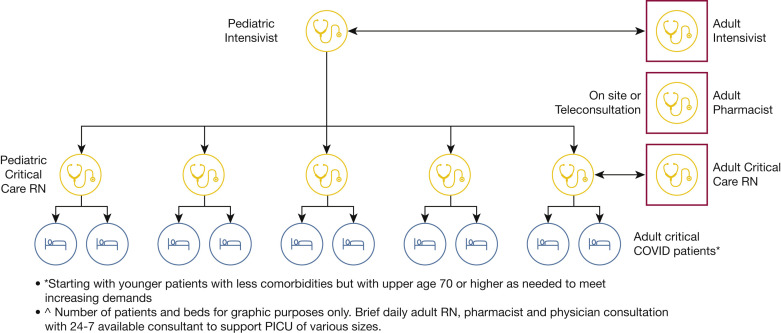
Figure 5.
Diagram showing model for staffing and support of PICUs embedded in facilities that routinely care for adult patients to support adult surge.32, 33, 34 Adult RNs, pharmacists, and physicians can provide brief daily and as-needed in-person or teleconsultation for pediatric ICUs of varying sizes providing care for critically ill adults starting with younger patients with less comorbidities, but with upper age of 70 years or older as needed to meet increasing demands. This model is supported most easily in facilities that also routinely care for adults because of existing logistic (“stuff”) and system capabilities. PICU = pediatric ICU; RN = registered nurse.
The Society of Critical Care Medicine’s tiered staffing model22 was believed to be appropriate for crisis level surge in larger institutions with sufficient added personnel; however, the model of a single supervisory intensivist overseeing care for up to 96 patients at contingency levels during COVID-19 is unrealistic. In updating staffing models, the TFMCC considered the limited published data and experience from facilities of different sizes experiencing at least one severe surge since March 2020. The three resulting staffing models (Figs 3, 4, 5) describe operational strategies to scale up surge staffing effectively and maintain contingency-level care.
Staffing should be adjusted based on surge severity, acuity, the experience of non-ICU clinicians, team compositions, available telemedicine support, and the duration that staff has been under strain. The physician ICU model consists of an intensivist (or other ICU-experienced physician) managing up to 24 patients in collaboration with non-ICU skilled physicians and advanced practice providers (including nurse practitioners, physician assistants, and similar professionals), delivering care to more patients than ICU staff could achieve alone (Fig 3). Further staffing can be expanded by use of specialized procedure teams (eg, for central venous catheterization, intubations, and assistance in care activities such as prone positioning) and telemedicine coverage.
The suggested nursing model expands the reach of one ICU-trained nurse to four patients by teaming with two nurses focused on the non-ICU aspects of care after a period of focused training (Fig 4). Both models account for staffing up to 100% above normal baseline patient levels, consistent with previous TFMCC suggestions for the upper limit of contingency care,4 but can be scaled proportionately to the level of surge. Achieving these ratios requires extensive clinical experience and may need to be modified for those with less training.
Pediatric ICUs can effectively care for adult patients with consultative support from adult teams, either in person or via telemedicine, mitigating the loss of time for training new staff in the basics of critical care medicine, while expanding system capacity due to lower pediatric ICU use experienced during the pandemic (Fig 5).32, 33, 34
Suggestion 2: We suggest limiting overtime to less than 50% above normal for all HCWs to minimize the risk of burn-out and exhaustion.
There are limits to how long a clinical team can remain effective in the setting of increased workloads. Experience with deployed military surgical teams found them to be ineffective after 48 continuous hours.23 Team effectiveness may be preserved by limiting shift durations to 12 h, mandating rest periods every 24 h, naps, “sleep banking,” and consistent schedules to prevent circadian desynchronization.23 Staffing plans must also account for surges of weeks to months. Limiting overtime should be an operational strategy to minimize the risk of burnout.
Suggestion 3: We suggest that the mental health needs of all HCWs are priorities for maintaining an effective response and staffing capacity.
HCWs are secondary victims of the COVID-19 pandemic.35 High proportions of HCWs have experienced acute stress disorders (40%), anxiety (30%), burnout (28%), depression (24%), posttraumatic stress disorder (13%),36 and suicide.37 Identification of factors that place HCWs at risk of moral injury or exhaustion (Table 2 ) should be a central operational priority, with a focus on prevention by leadership, managing expectations, and proactive guidance for changes to clinical care.36 , 38, 39, 40, 41, 42, 43
Table 2.
Risk Factors for Health Care Workers Experiencing Moral Injury or Exhaustion36,38, 39, 40, 41, 42, 43
| Work environment |
|---|
| • Inadequate access to personal protective equipment or essential supplies |
| • High perception of personal risk for infection |
| • Inability to rest |
| • Prolonged working times |
| • Excessive workload |
| • Working in a high-risk environment |
| • Involuntary deployment |
| • Perceived inadequate training |
| • Lack of sufficient communication and updated information |
| • Regret about restricted visitation policies |
| • Witnessing hasty end-of-life decisions |
| • Inadequate organizational support, insurance, or compensation |
| Social factors |
|---|
| • Fear of being infected |
| • Fear of spreading the illness to family and friends |
| • Inability to care for one’s family |
| • Struggling with difficult emotions |
| • Being quarantined |
| • Social rejection or isolation |
| • Moral distress |
| • Lack of social support |
Globally, the health care workforce is composed predominantly of women in diverse roles, including critical custodial, food service, laboratory, and radiographic technicians and direct patient care by nurses, advanced practice providers, and physicians. Of nurses in the United States, 87.4% are women who may find themselves bearing a disproportionate amount of the stresses of both workplace and home during the pandemic.44 , 45 A survey of > 12,000 US nurses reported that workplace strategies that best promoted well-being during the pandemic included talking with colleagues (47%), expressions of gratitude (37%), and accurate information about the virus (34%)46; only 5% identified employee assistance programs or formal counseling as helpful.47 The Healthy Work Environment Standards (Fig 6 ) created by the American Association of Critical Care Nurses provide a framework for operational strategies to promote resilience and well-being in times of surge (Fig 6, Table 3 ).46 Concerns such as childcare, reduced family contact, significant change in daily life, and work-life balance place individuals at high risk for burnout.36 , 38, 39, 40, 41, 42, 43
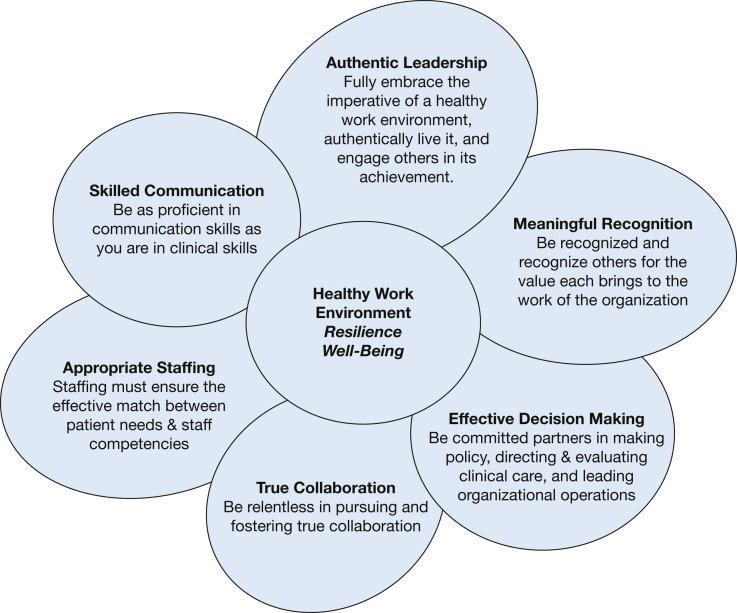
Figure 6.
Diagram showing the American Association of Critical Care Nurses Healthy Work Environment Standards.46 The six standards have been shown to correlate with burnout among critical care nurses. The Healthy Work Environment Standards have been demonstrated to apply to the interdisciplinary team and to noncritical care areas. Authentic leadership and meaningful recognition are shown to correlate most strongly with compassion satisfaction, which counteracts compassion fatigue and enhances professional quality of life.46
Table 3.
Resilience Strategies During Surge Capacity Using the Healthy Work Environment Framework46, 47, 30, 31, 48, 49, 50, 51
| Standard | Implementation Strategies During Surge |
|---|---|
| Skilled communication |
|
| Authentic leadership |
|
| Meaningful recognition |
|
| Appropriate staffing | |
| Effective decision-making |
|
| True collaboration | Use just-in-time strategies for supporting the interdisciplinary team:
|
PPE = personal protective equipment.
Suggestion 4: During surge, we suggest minimizing redundant clinical documentation requirements to focus on core elements directly relevant to bedside care.
The TFMCC believes it is an effective operational strategy to reduce documentation requirements to maximize staff time for bedside care during public health emergencies. Clinician documentation should focus mainly on critical care provided and limitations due to resource challenges and should address issues related to diagnoses for billing; templated notes and dictation services are supportive. Nursing documentation changes may include exemptions from repetition of documented care plans or extended time windows for signatures on telephone orders.52 Streamlining facility-specific documentation and focused patient assessments may further assist in decreasing documentation requirements. Authorities should provide general approvals for streamlined documentation during a public health emergency to help facilities avoid the burden of requesting individual waivers.
Load-Balancing and Patient Transfers
Load-balancing is the process of coordinating emergency response by sharing resources, transferring patients amongst hospitals, or both.53, 54, 55, 56 This concept became imperative during COVID-19 surges, with hospital mortality increasing when bed capacity was exceeded.6 , 7 , 57 , 58 Effective load-balancing first involves knowledgeable ICU strain monitoring and mindful resource management by frontline clinical leaders. It next involves the organized transfer of patients from overburdened to lesser burdened hospitals facilitating a balance, so that no single institution enters crisis and that consistent level of care is provided within and across regions. Patient transfers within a single health care network are helpful, but insufficient. Regional mechanisms to share information and load-balance across facilities may be the most important factor in mitigating crisis care situations.59, 60, 61
Suggestion 5: We suggest that resource strain level be actively monitored and determined by frontline clinical leaders based upon assessment of available resources and conditions.
ICU strain is defined as a discordance between demand for and availability of ICU resources and is assessed with objective criteria.62 , 63 Table 4 suggests ICU strain indicators that are resources ICU clinicians and leaders assess during routine ICU management and include staffed ICU beds, patient acuity, queuing time to admission, available equipment, supplies, and ICU staff.1 , 4 , 5 , 56 , 63 , 64 Strain is defined further based on conventional, contingency, and crisis care surge levels. The TFMCC suggests the criteria for crossing from conventional to contingency threshold be when two or more conventional strain criteria are exceeded and the criteria for crossing form contingency to crisis be when any crisis strain criterion is met (Table 4). We suggest strain indicators be updated continuously and available in an electronic database.
Table 4.
Strain Indicator Limits Defining Conventional, Contingency, and Crisis Levels of Resources Typically Encountered During the COVID-19 Pandemic1,4,5,56,63,64,65
| Variable | Conventional Strain Criteria With Upper Limits | Contingency Strain Criteria | Crisis Strain Criteria |
|---|---|---|---|
| ICU beds (“space”) |
|
|
|
| Equipment, oxygen, and medications (“stuff”) |
|
Equipment, oxygen, and medications sufficient for routine standard of care, but may need to adapt, substitute, or conserve:
|
Equipment, oxygen, and medications not sufficient to maintain routine standard of care and care adapted, rationed, or triaged with substantial patient risk:
|
| Staff |
|
|
|
The transition from conventional to contingency strain is triggered when any two conventional strain indicators are exceeded (any "no" answer in the conventional strain criteria column should be considered to have exceeded that strain criteria's conventional limit). The transition from contingency to crisis strain is triggered when any crisis strain criteria is met. CRRT = continual renal replacement therapy; HD = hemodialysis; HFNC = high flow nasal cannula; NIV = noninvasive ventilation; RN = registered nurse.
As an operational strategy, the TFMCC suggests bedside clinician leaders, including ICU directors and service chiefs, be empowered to determine ICU surge level (especially contingency and crisis) based on their real-time assessment and in conjunction with strain indicators (Table 4, Fig 7 ). Hospital and system leadership may sustain contingency-level care by expanding ICU care areas, supplementing staff, and distributing equipment, transferring patients to load-balance, or a combination thereof.
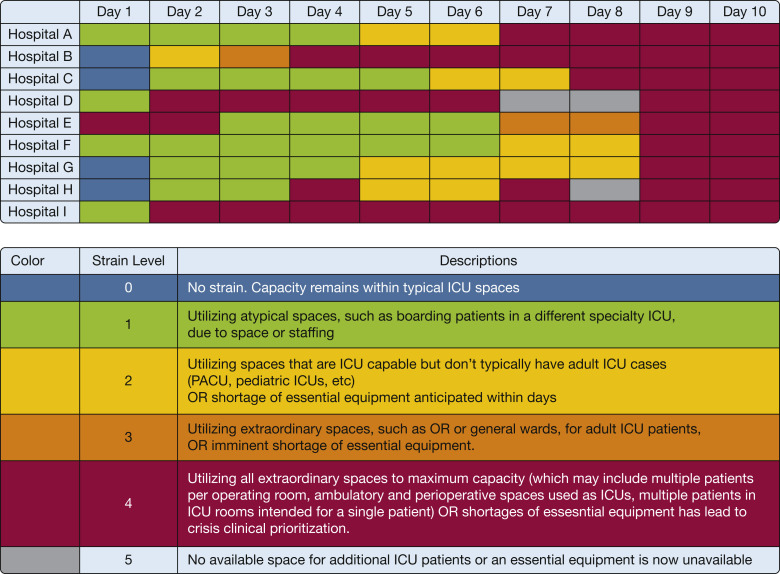
Figure 7.
Diagram showing an example of a nine-hospital system illustrating daily levels of strain during a severe pandemic surge. Using clinical leaders’ assessment and strain criteria each of nine hospitals (left margin, top frame) assesses their daily level of ICU surge for the first 10 days (shown across the top); the levels of daily strain are illustrated in the colored legend below. For health system leadership, the ebb and flow of daily level of strain for each hospital helps to determine where hospital resources need to be directed, where resources are available to transfer patients, or both. PACU = post-anesthesia care unit.
Suggestion 6: We suggest there is a transition zone toward the limits of contingency care when increasingly scarce resources are modified beyond routine standards of care to preserve life. This critical clinical prioritization level precedes triage of scarce resources and is a powerful indicator for needed resources to maintain contingency-level care. 54 (Case study Fig 8 )
Figure 8.
Case study of CCP. CCP = critical clinical prioritization.
Although guidance exists for formal triage, clinicians may encounter situations of severe strain that fall short of this need, where clinical judgement is needed to determine optimal use of available resources.12 , 55 , 63 The use of advanced therapies is not strictly binary; resources may be shared in some cases. Continual renal replacement therapy systems can be shared by two or more patients on 6- to 12-h alternating schedules; the choice of ventilator for a given patient can be based on the severity of lung disease; and patients with severe hypoxemia often may be managed with noninvasive respiratory support in an intermediate or ward setting instead of the ICU. These strategies are a form of resource conservation, but border on crisis care and are termed critical clinical prioritization (CCP) (Fig 9); a case study is illustrative (Fig 8).54 , 56 , 66
Figure 9.
Diagram showing critical clinical prioritization. As resource strain approaches crisis levels, ICU clinicians may need to adapt, substitute, conserve, or even initiate rationing of resources.56 This transition zone immediately preceding crisis level is termed critical clinical prioritization and is illustrated on the lower panel, “Basis of Clinical Management.”
Clinicians should recognize CCP when they are on the verge of transitioning from contingency to crisis and should alert leadership and request urgent support, including more resources (if available) or patient transfers (load-balance) to less-strained facilities. If neither of these are available, health systems are left resorting to triage and allocation processes dynamically based on available resources. Ideally, these strategies should be consistent across a region’s hospitals.
Operational strategies include educating clinicians in advance to recognize and respond to CCP, preparing decision support for potential crisis scenarios, and prioritizing communication systems for rapid access to ethical, legal, and administrative counsel when the potential need for triage of scarce resources is encountered.
Suggestion 7: We suggest that early transfer of patients before a hospital is overwhelmed promotes the effective conservation of resources and less deviation from routine care standards.
The mortality of patients rises as pandemic surge increases. Early transfer of patients is an operational strategy to help mitigate proactively the effects of surge and to prevent crisis care conditions.10 , 67 Patients awaiting ICU admission have an increased mortality with longer-duration queuing times, and patients in the ED are at great risk (Table 5 ).63 , 68, 69, 70 Strategies to mitigate risks during transfer include assuring respiratory and hemodynamic stability and transferring patients with minimal organ failure and at shorter times and distances as possible (Table 5).63 , 68, 69, 70 We suggest patient transfer be considered when hospitals reach the threshold for contingency care surge level (suggestion 5).
Table 5.
Risk for Patients Waiting for ICU Admission vs the Risks of Transfer, and Strategies to Help Mitigate Patient Transfer Risk During Pandemic Surge63,68, 69, 70,71
| Risk for Patients Awaiting ICU Admission (≥ 6 h) | Risk for Patients During Transfer | Strategies to Mitigate Transfer Risk |
|---|---|---|
Increased risk for persistent organ dysfunction or death:
|
|
|
PACU = post-anesthesia care unit.
Despite transfer risks, load-balancing before a site becomes overwhelmed, including the transfer of convalescent patients to less-acute settings, promotes effective conservation of resources before reaching the limits of contingency care.6 , 10 , 67 Conversely, the failure to initiate transfers may be associated with greater deviation from standards of care and with increased morbidity, along with the urgent need to transfer greater numbers of sicker patients quickly when crisis conditions do occur.72
Suggestion 8: We suggest earlier utilization of regional transfer centers for load-balancing during surge for patient transfers and placement. We also suggest having intensivist or hospitalist availability to help prioritize transfers and provide support to bedside clinicians when transfers are delayed. 73
In the TFMCC 2014 guidance, evacuation of hospitals for surge mitigation was highlighted74; however, hospitals have been challenged by management of patient transfers during prolonged surges. The COVID-19 pandemic demonstrated increasing mortality associated with increasing surge,6 , 10 and load-balancing through large-volume patient transfer centers has been proven both practical and effective.59 , 60 , 75
Health systems have developed their own regional placement centers designed to improve access and patient flow through active management techniques.76, 77, 78 Several states rapidly developed pandemic placement centers to transfer patients to any available ICU bed, optimizing statewide access and preventing hospitals from reaching crisis conditions.59, 60, 61 , 79 The operational strategy of having a command center is to improve access and assure efficient use of beds through active management and load-balancing of admissions across all hospitals to reduce ED boarding and diversion and to prevent extended waiting times for admission.80
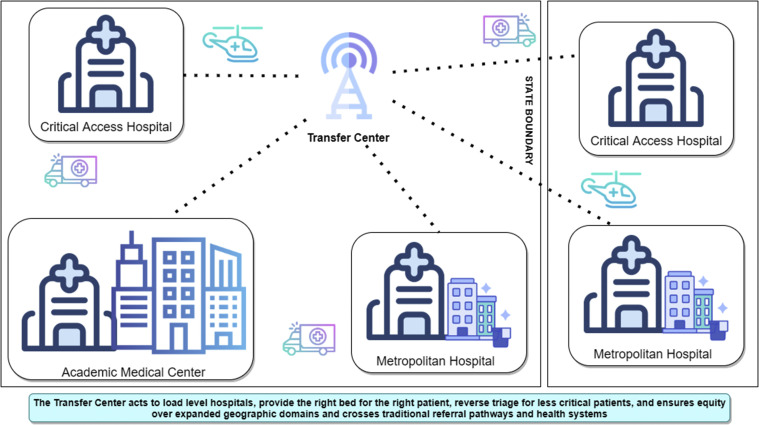
The key elements of success include a call center with appropriate technology and personnel, participation of large health systems with transfer capacity, and agreements by participants to support the transfer center’s processes with clear lines of communication between call center directors and state or health system leaders to resolve barriers rapidly (Fig 10 ). We also strongly suggest having intensivist or hospitalist assistance to help prioritize transfers and to provide assistance to bedside clinicians.
Figure 10.
Diagram showing regional and statewide patient placement centers. Transfer centers interface with all hospital and health systems in a region or state and typically may be engaged after routine referral sources are no longer accepting transfers. Their role is to facilitate patient transfers quickly to an appropriate hospital setting including ICU and medical or surgical beds, while efficiently and effectively using capacity at both larger and smaller hospitals. The ability to pay should never be a criterion regarding transfer, and transfer centers should have policy authority to rotate transfers if required. Transfer distances may require a combination of ground and air transport. Intensivists and hospitalists may help to prioritize transfers based on both the type of (specialized) care needed and urgency of transfer, and they may be able to provide clinical advice to onsite clinicians whose patients may not be able to be transferred immediately.
Best practices are under development. Technology from cell phones and paper intake forms should transition to secure electronic forms and telephone platforms. Oregon,79 Arizona,60 and Minnesota59 use tools including electronic bed boards to report bed availability and calls requesting transfers. The most important success element was frequently updated electronic bed capacity data (even when hand-entered), although collaboration and teamwork among call center, state, and health care organizations is as important.59 , 60 , 79
Communications During Surge
Suggestion 9: We re-emphasize that designated clinicians who are actively engaged in clinical work (especially intensivists and hospitalists) actively participate in hospital incident command structure; this group should provide updates to clinical staff for improving situational awareness, ensuring bidirectional communication.
As stated in 2014, tight coordination of resource management is a cornerstone of effective hospital incident command systems.4 , 81 Although a hospital incident command system functions as the primary decision-maker with authority to act quickly, the actions occurring in incident command often are opaque to HCWs tasked with their orders. It is strongly suggested as an operational strategy that formal communication structures be established to ensure bidirectional communication to update clinicians and provide decisive clinical feedback to hospital and health system leaders (suggestion 5).
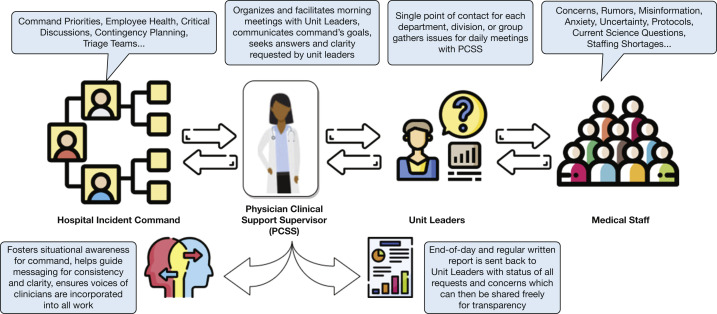
We suggest the use of a formal hospital incident command system position, identified as the physician clinical support supervisor or physician clinical support supervisor team.82 , 83 Physician clinical support supervisors are actively engaged and present with both their clinical colleagues providing care and within incident command center functions. Although division or department heads may fill this role, experience suggests respected clinical leaders are likely most effective. Town hall-type meetings, tiered huddles, and structured e-mails are other important communication vehicles (Figure 11 ).
Figure 11.
Diagram showing the role of the PCSS. PCSS = physician clinical support supervisor.
Technological Issues and Solutions During Surge Management
Suggestion 10: We suggest hospitals apply telemedicine technology to augment critical care early and in the broadest sense possible.
Telemedicine was used to augment surge capacity and to provide family access for communication with hospitalized patients using straightforward and inexpensive technology including computers, electronic tablets, and conferencing software. Health care organizations used this technology to connect intensivists and specialists to distant rural sites and potentially to tele-triage.84 , 85 Outpatient strategies include monitoring at-risk but stable patients remotely, home care for stable patients (hospital-at-home programs), and outpatient care (Fig 12 ),86, 87, 88 thereby helping to decompress hospital surge. The TFMCC strongly suggests that this technology is a powerful adjunct to providing care in less resourced environments, although licensing and credentialing issues must be addressed quickly in an emergency.
Figure 12.
Diagram showing telemedicine and tele-ICU technology. Telemedicine and tele-ICU technology with portable applications can augment both the delivery of clinical expertise to virtually any hospital bedside in support of ICU care and specialty consultation support and can provide families virtually unlimited audiovisual access to their loved ones who are hospitalized patients (right side of diagram). This technology also functions by helping decompress hospital surge by outpatient care reach support for patients at home and preventing disease exposure from unnecessary office visits (left side of diagram).
Initially, visitation was prohibited during the pandemic, which limited contributions from loved ones to care and provide decision-making goals,89 , 90 and telemedicine was used to support the needs of families and patients. Operational strategies to facilitate family presence in the ICU includes video conferencing and conventional telephones with regular inclusion of multidisciplinary team members such as psychiatrists, palliative care specialists, social workers, and chaplains. Visitation policies also should include on-site strategies for families with limited access to technology
Discussion
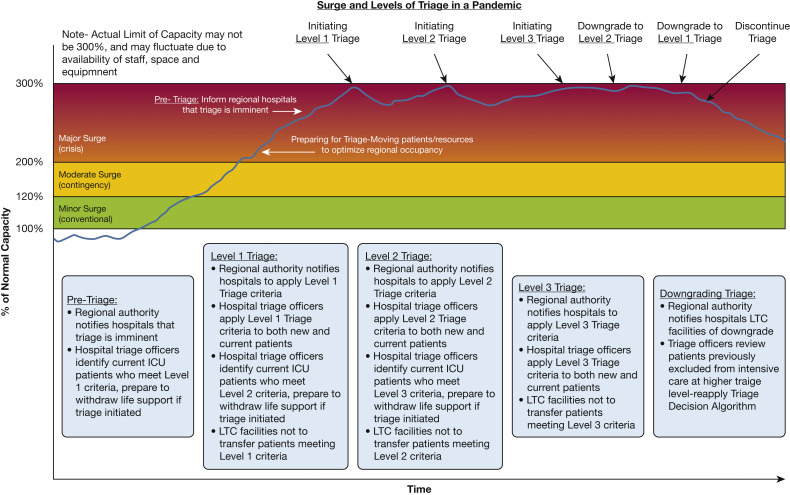
Before the COVID-19 pandemic, mass critical care guidelines were tested infrequently under contingency or crisis conditions, and thus were underappreciated by planners. The impact of a prolonged public health emergency, leading to a state of chronic contingency care that would fluctuate unpredictably (Fig 13 ),12 has resulted in significant workforce stress. With increasing surge, mortality rises—sometimes dramatically—and it is likely many of our health care systems endured a chronic state of crisis conditions.2 , 6 , 10
Figure 13.
Diagram showing the impact of triage in crisis surge response to balance demand and capacity, demonstrating different levels of triage depending on the degree of demand in relationship to system capacity. LTC = long-term care. (Reprinted with permission from Maves et al.12)
ICU clinicians recognized a highly dynamic zone of contingency care and intuitively developed ingenious strategies to identify and extend these boundaries, and the TFMCC focused on greater understanding of this contingency zone.
Sustaining a large operational workforce was a top priority. The three staffing strategies incorporate critical care-trained clinicians working with other professionals, combining skill sets and procedure teams and leveraging telemedicine ICU support and adult care in pediatric ICUs in needed proportions to sustain contingency-level care. They are adaptable and expandable based on surge levels. Also essential is supporting HCW resilience over months, including a reasonable ceiling of work hours, responsibly limiting required documentation, and addressing HCW’s mental health needs through effective communication, adapting quickly to surge demands and promoting a healthy work environment (Tables 2, 3, Fig 6).
Effectively managing surge resources and load-balancing is another priority. ICU pandemic strain was experienced poignantly by ICU clinician leaders, and their understanding of staff, bed, and supply resources in absolute amounts and imminent availability impacts the degree to which resources may be stretched to responsible limits during periods of CCP. The TFMCC strongly suggests clinical leadership be empowered to determine surge level and priorities, with the suggested communication strategies (suggestion 9) pivotal in keeping clinicians and administrative leaders aligned.
ICU strain indicators (Table 4) are suggested as adjuncts for clinician leader assessment in determining surge levels and priorities, are useful under routine conditions, and can be acquired electronically for ongoing use and predictive analysis. At present, ICU queuing time may be the most powerful strain indicator, given its strong correlation with mortality (Table 5),63 and among the most compelling reasons for early load-balancing to prevent overcrowding and crisis care during times of severe surge.6 , 10
Finally, transfer hubs proved an instrumental strategy in load-balancing hospitals at or near crisis to those with remaining capacity and a powerful process for maintaining contingency level care across regions and states, ranging from statewide support for individual centers to massive regional off-loading of specific sites.59 , 75 Successful transfer centers demand relatively unsophisticated technology and software, but as a top priority require regional and statewide commitment, coordination, and above all teamwork among health care systems, professional associations, and health departments.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. T. H. and R. C. M. are United States government employee or military service member. This work was prepared as part of his official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties. The opinions and assertions contained herein are those of the authors and do not reflect the official views or position of the Department of the Army, Department of Defense, Department of Veterans Affairs, the United States Government, nor of the academic institutions with which the authors are affiliated. None declared (J. R. D., A. V. D., C. L. S., V. M., J. P., K. D. B., D. O., A. U., T. H., K. N. H., M. G., K. R. B., H. J. F., L. D. B., A. M. O. M., M. H., P. K. T., J. D., J. L. H., M. D. C.).
∗The Task Force for Mass Critical Care Writing Group: Anwar Al-Awadhi, Timur Alptunaer, Marie Baldisseri, Wanda Barfield, Karyn D. Baum, Joshua Benditt, Kasey Bowden, Richard Branson, Lisa D. Burry, Michael Christian, Asha V. Devereaux, Jeffrey R. Dichter, Guillermo Dominguez-Cherit, James Downar, David Dries, Sharon Einav, Mill Etienne, Laura Evans, Henry J. Feldman, James Geiling, Marya Ghazipura, Ramon Gist, Kelly Griffin, Neil Halpern, Mitchell T. Hamele, Kiersten Henry, Attila Hertelendy, John Hick, Meredith Huffines, Nathaniel Hupert, Tanzib Hossain, David Ingbar, Sameer S. Kadri, Sarah Kesler, Mary A. King, Niranjan Kissoon, Kristi Koenig, Joseph Lamana, Lindsay Leif, Deborah Levy, Alicia Livinsky, Christie Martin, Anne Marie Martland, Ryan C. Maves, Steven Mitchell, Vikramjit Mukherjee, Mangala Narasimhan, Alexander Niven, Juan Ochoa, Doug Ornoff, J. Scott Parrish, Jason Persoff, Tia Powell, MJ Reed, Dario Rodriguez, Gilbert Seda, Jaspal Singh, Julie Solar, Charles L. Sprung, Eric Toner, Pritish K. Tosh, Amit Uppal, and Marian Von-Maszewski.
Other contributions: The authors thank Adam V. Meyer, MD, Assistant Professor, University of Colorado School of Medicine, for his contribution to the provider staffing graphic; and the ICU directors across the NYC Health and Hospitals Network, led by Leon Boudourakis, MD, NYC Health + Hospitals/Kings County Medical Center, and Matthew Langston, MD, Department of Medicine, NYC Health and Hospitals/Jacobi, for their heroic clinical efforts and leadership throughout the pandemic and for which much of this work would not otherwise have been possible.
Additional information: The e-Appendix can be found in the Supplemental Materials section of the online article.
Footnotes
DISCLAIMER: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at https://www.chestnet.org/Guidelines-and-Resources.
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Contributor Information
Task Force for Mass Critical Care Writing Group:
Anwar Al-Awadhi, Timur Alptunaer, Marie Baldisseri, Wanda Barfield, Karyn D. Baum, Joshua Benditt, Kasey Bowden, Richard Branson, Lisa D. Burry, Michael Christian, Asha V. Devereaux, Jeffrey R. Dichter, Guillermo Dominguez-Cherit, James Downar, David Dries, Sharon Einav, Mill Etienne, Laura Evans, Henry J. Feldman, James Geiling, Marya Ghazipura, Ramon Gist, Kelly Griffin, Neil Halpern, Mitchell T. Hamele, Kiersten Henry, Attila Hertelendy, John Hick, Meredith Huffines, Nathaniel Hupert, Tanzib Hossain, David Ingbar, Sameer S. Kadri, Sarah Kesler, Mary A. King, Niranjan Kissoon, Kristi Koenig, Joseph Lamana, Lindsay Leif, Deborah Levy, Alicia Livinsky, Christie Martin, Anne Marie Martland, Ryan C. Maves, Steven Mitchell, Vikramjit Mukherjee, Mangala Narasimhan, Alexander Niven, Juan Ochoa, Doug Ornoff, J. Scott Parrish, Jason Persoff, Tia Powell, M.J. Reed, Dario Rodriguez, Gilbert Seda, Jaspal Singh, Julie Solar, Charles L. Sprung, Eric Toner, Pritish K. Tosh, Amit Uppal, and Marian Von-Maszewski
Supplementary Data
References
- 1.Griffin K.M., Karas M.G., Ivascu N.S., Lief L. Hospital preparedness for COVID-19: a practical guide from a critical care perspective. Am J Respir Crit Care Med. 2020;201(11):1337–1344. doi: 10.1164/rccm.202004-1037CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christian M.D., Devereaux A.V., Dichter J.R., et al. Introduction and executive summary: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4 suppl):8S–34S. doi: 10.1378/chest.14-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hick J.L., Einav S., Hanfling D., et al. Surge capacity principles: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4 suppl):e1S–e16S. doi: 10.1378/chest.14-0733. [DOI] [PubMed] [Google Scholar]
- 5.Einav S., Hick J.L., Hanfling D., et al. Surge capacity logistics: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4 suppl):e17S–e43S. doi: 10.1378/chest.14-0734. [DOI] [PubMed] [Google Scholar]
- 6.Bravata D.M., Perkins A.J., Myers L.J., et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.34266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubinson L. Intensive care unit strain and mortality risk among critically ill patients with COVID-19: there is no “me” in COVID. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.35041. [DOI] [PubMed] [Google Scholar]
- 8.Bader M.K., Braun A., Fox C., et al. A California hospital’s response to COVID-19: from a ripple to a tsunami warning. Crit Care Nurse. 2020;40(6):e1–e16. doi: 10.4037/ccn2020799. [DOI] [PubMed] [Google Scholar]
- 9.Burry L.D., Barletta J.F., Williamson D., et al. It takes a village . . . : contending with drug shortages during disasters. Chest. 2020;158(6):2414–2424. doi: 10.1016/j.chest.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadri S.S., Sun J., Lawandi A., et al. Association between caseload surge and COVID-19 survival in 558 U.S. hospitals, March to August 2020. Ann Intern Med. 2021;174(9):1240–1251. doi: 10.7326/M21-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lost on the Frontline. Our key findings about US healthcare worker deaths in the pandemic’s first year: the project counted more than 3,600 healthcare worker deaths, with the majority of people who died under the age of 60. The Guardian. April 8, 2021 https://www.theguardian.com/us-news/ng-interactive/2020/dec/22/lost-on-the-frontline-our-findings-to-date Accessed May 5, 2021. [Google Scholar]
- 12.Maves R.C., Downar J., Dichter J.R., et al. Triage of scarce critical care resources in COVID-19: an implementation guide for regional allocation: an expert panel report of the Task Force for Mass Critical Care and the American College of Chest Physicians. Chest. 2020;158(1):212–225. doi: 10.1016/j.chest.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garritty C.M., Norris S.L., Moher D. Developing WHO rapid advice guidelines in the setting of a public health emergency. J Clin Epidemiol. 2017;82:47–60. doi: 10.1016/j.jclinepi.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan R.L., Florez I., Falavigna M., et al. Development of rapid guidelines: 3. GIN-McMaster Guideline Development Checklist extension for rapid recommendations. Health Res Policy Syst. 2018;16(1):63. doi: 10.1186/s12961-018-0330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ornelas J., Dichter J.R., Devereaux A.V., et al. Methodology: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4 suppl):35S–41S. doi: 10.1378/chest.14-0746. [DOI] [PubMed] [Google Scholar]
- 16.Jaeschke R., Guyatt G.H., Dellinger P., et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;337:a744. doi: 10.1136/bmj.a744. [DOI] [PubMed] [Google Scholar]
- 17.Lewis S.Z., Diekemper R., Ornelas J., Casey K.R. Methodologies for the development of CHEST guidelines and expert panel reports. Chest. 2014;146(1):182–192. doi: 10.1378/chest.14-0824. [DOI] [PubMed] [Google Scholar]
- 18.Assistant Secretary for Preparedness and Response, United States Department of Health and Human Services Mass critical care: system level surge staffing and resilience strategies Project ECHO clinical rounds February 2, 2021. https://www.youtube.com/watch?v=Jko_I1NlFvE
- 19.Assistant Secretary for Preparedness and Response, United States Department of Health and Human Services Critical care: successful staffing models for severe pandemic surge: current update. Project ECHO clinical rounds March 16, 2021. https://www.youtube.com/watch?v=GAdUm5NMydQ
- 20.Rosenbaum L. Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873–1875. doi: 10.1056/NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 21.Sprung C.L., Joynt G.M., Christian M.D., Truog R.D., Rello J., Nates J.L. Adult ICU triage during the coronavirus disease 2019 pandemic: who will live and who will die? Recommendations to improve survival. Crit Care Med. 2020;48(8):1196–1202. doi: 10.1097/CCM.0000000000004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halpern N.A., Kay S.T. United States resource availability for COVID-19. May 12, 2020. Society of Critical Care Medicine website. https://www.sccm.org/getattachment/Blog/March-2020/United-States-Resource-Availability-for-COVID-19/United-States-Resource-Availability-for-COVID-19.pdf?lang=en-US
- 23.Parker R.S., Parker P. The impact of sleep deprivation in military surgical teams: a systematic review. J R Army Med Corps. 2017;163(3):158–163. doi: 10.1136/jramc-2016-000640. [DOI] [PubMed] [Google Scholar]
- 24.Aly S., Talutis S.D., Richman A.P., et al. The Boston Medical Center Coronavirus Disease 2019 (COVID-19) Procedure Team: optimizing the surgeon’s role in pandemic care at a safety-net hospital. Surgery. 2020;168(3):404–407. doi: 10.1016/j.surg.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nawathe P., Wong R., Pollock G., et al. Creation of a dedicated line team for critically ill patients with COVID-19: a multidisciplinary approach to maximize resource utilization during the COVID-19 pandemic. J Vasc Access. 2021 doi: 10.1177/1129729821991754. 1129729821991754. [DOI] [PubMed] [Google Scholar]
- 26.Chun T.T., Judelson D.R., Rigberg D., et al. Managing central venous access during a health care crisis. J Vasc Surg. 2020;72(4):1184–1195 e1183. doi: 10.1016/j.jvs.2020.06.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall A.P., Austin D.E., Chamberlain D., et al. A critical care pandemic staffing framework in Australia. Aust Crit Care. 2021;34(2):123–131. doi: 10.1016/j.aucc.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oakley C., Pascoe C., Balthazor D., et al. Assembly line ICU: what the long shops taught us about managing surge capacity for COVID-19. BMJ Open Qual. 2020;9(4):e001117. doi: 10.1136/bmjoq-2020-001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathews K.S., Seitz K.P., Vranas K.C., et al. Variation in initial U.S. hospital responses to the coronavirus disease 2019 pandemic. Crit Care Med. 2021;49(7):1038–1048. doi: 10.1097/CCM.0000000000005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassidy L. Team nursing and COVID-19 surge staffing. May 5, 2020. American Association of Critical-Care Nurses website. https://www.aacn.org/blog/team-nursing-and-covid-19-surge-staffing
- 31.Martland A., Huffines M., Henry K. Surge priority planning COVID-19: critical care staffing and nursing considerations. 2020. American College of Chest Physicians website. http://www.chestnet.org/Guidelines-and-Resources/Resources/Surge-Priority-Planning-COVID-19-Critical-Care-Staffing-and-Nursing-Considerations
- 32.Zee-Cheng J., McCluskey C.K., Klein M.J., et al. Changes in pediatric ICU utilization and clinical trends during the coronavirus pandemic. Chest. 2021;160(2):529–537. doi: 10.1016/j.chest.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joyce C.L., Howell J.D., Toal M., et al. Critical care for coronavirus disease 2019: perspectives from the PICU to the medical ICU. Crit Care Med. 2020;48(11):1553–1555. doi: 10.1097/CCM.0000000000004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deep A., Knight P., Kernie S.G., et al. A hybrid model of pediatric and adult critical care during the coronavirus disease 2019 surge: the experience of two tertiary hospitals in London and New York. Pediatr Crit Care Med. 2021;22(2):e125–e134. doi: 10.1097/PCC.0000000000002584. [DOI] [PubMed] [Google Scholar]
- 35.Karnatovskaia L.V., Johnson M.M., Varga K., et al. Stress and fear: clinical implications for providers and patients (in the time of COVID-19 and beyond) Mayo Clin Proc. 2020;95(11):2487–2498. doi: 10.1016/j.mayocp.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serrano-Ripoll M.J., Meneses-Echavez J.F., Ricci-Cabello I., et al. Impact of viral epidemic outbreaks on mental health of healthcare workers: a rapid systematic review and meta-analysis. J Affect Disord. 2020;277:347–357. doi: 10.1016/j.jad.2020.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman A., Plummer V. COVID-19 related suicide among hospital nurses; case study evidence from worldwide media reports. Psychiatry Res. 2020;291:113272. doi: 10.1016/j.psychres.2020.113272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azoulay E., Cariou A., Bruneel F., et al. Symptoms of anxiety, depression, and peritraumatic dissociation in critical care clinicians managing patients with COVID-19. A cross-sectional study. Am J Respir Crit Care Med. 2020;202(10):1388–1398. doi: 10.1164/rccm.202006-2568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobson H., Malpas C.B., Burrell A.J., et al. Burnout and psychological distress amongst Australian healthcare workers during the COVID-19 pandemic. Australas Psychiatry. 2021;29(1):26–30. doi: 10.1177/1039856220965045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Hage W., Hingray C., Lemogne C., et al. [Health professionals facing the coronavirus disease 2019 (COVID-19) pandemic: what are the mental health risks?] Encephale. 2020;46(3S):S73–S80. doi: 10.1016/j.encep.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stuijfzand S., Deforges C., Sandoz V., et al. Psychological impact of an epidemic/pandemic on the mental health of healthcare professionals: a rapid review. BMC Public Health. 2020;20(1):1230. doi: 10.1186/s12889-020-09322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoruk S., Guler D. The relationship between psychological resilience, burnout, stress, and sociodemographic factors with depression in nurses and midwives during the COVID-19 pandemic: a cross-sectional study in Turkey. Perspect Psychiatr Care. 2021;57(1):390–398. doi: 10.1111/ppc.12659. [DOI] [PubMed] [Google Scholar]
- 43.Raudenska J., Steinerova V., Javurkova A., et al. Occupational burnout syndrome and post-traumatic stress among healthcare professionals during the novel coronavirus disease 2019 (COVID-19) pandemic. Best Pract Res Clin Anaesthesiol. 2020;34(3):553–560. doi: 10.1016/j.bpa.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boniol M., McIsaac M., Xu L., Wuliji T., Diallo K., Campbell J. Gender equity in the health workforce: analysis of 104 countries. Health Workforce Working Paper 1. World Health Organization. 2019. World Health Organization website. https://apps.who.int/iris/bitstream/handle/10665/311314/WHO-HIS-HWF-Gender-WP1-2019.1-eng.pdf
- 45.United States Bureau of Labor Statistics Labor force statistics from the current population survey. 2020. Bureau of Labor Statistics website. https://www.bls.gov/cps/cpsaat11.htm
- 46.Monroe M., Morse E., Price J.M. The relationship between critical care work environment and professional quality of life. Am J Crit Care. 2020;29(2):145–149. doi: 10.4037/ajcc2020406. [DOI] [PubMed] [Google Scholar]
- 47.American Nurses Foundation Pulse on the nation’s nurses COVID-19 survey serious: mental health and wellness. American Nurses’ Foundation website. https://www.nursingworld.org/practice-policy/work-environment/health-safety/disaster-preparedness/coronavirus/what-you-need-to-know/mental-health-and-wellness-survey-2/
- 48.Adair K., Kennedy L., Sexton J. Three good tools: positively reflecting backwards and forwards is associated with robust improvements in well-being across three distinct interventions. J Posit Psychol. 2020;15(5):613–622. doi: 10.1080/17439760.2020.1789707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connor J.A., Ziniel S.I., Porter C., et al. Interprofessional use and validation of the AACN healthy work environment assessment tool. Am J Crit Care. 2018;27(5):363–371. doi: 10.4037/ajcc2018179. [DOI] [PubMed] [Google Scholar]
- 50.Good V. Healthy work environments in a crisis. Webinar Series 2021. American Association of Critical-Care Nurses website. https://www.aacn.org/education/webinar-series/wb0063/healthy-work-environments-in-a-crisis
- 51.Yount R.A., Olmert M.D., Lee M.R. Service dog training program for treatment of posttraumatic stress in service members. US Army Med Dep J. 2012;Apr-Jun:63–69. [PubMed] [Google Scholar]
- 52.Centers for Medicare and Medicaid Services, United States Department of Health and Human Services COVID-19 emergency declaration blanket waivers for health care providers 2021. Centers for Medicare and Medicaid Services website. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
- 53.Assistant Secretary for Preparedness and Response, United States Department of Health and Human Services Critical care: indicators for “ramping up and ramping down” surge capacity: balancing pandemic surge vs. versus the need to resume normal operations. Project ECHO clinical rounds March 9, 2021. https://www.youtube.com/watch?v=cvjvpVn88TQ
- 54.Assistant Secretary for Preparedness and Response, United States Department of Health and Human Services Triage of scarce resources version 2.0. Project ECHO clinical rounds March 23, 2021. https://www.youtube.com/watch?v=Zf5db2PhCNs
- 55.Christian M.D., Sprung C.L., King M.A., et al. Triage: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4 suppl):e61S–e74S. doi: 10.1378/chest.14-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hick J.L., Barbera J.A., Kelen G.D. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Prep. 2009;3(2 suppl):S59–S67. doi: 10.1097/DMP.0b013e31819f1ae2. [DOI] [PubMed] [Google Scholar]
- 57.Kohn R. Broadening the scope of healthcare operations: expanding capacity strain hospital-wide. Crit Care Med. 2020;48(5):771–773. doi: 10.1097/CCM.0000000000004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilcox M.E., Harrison D.A., Patel A., Rowan K.M. Higher ICU capacity strain is associated with increased acute mortality in closed ICUs. Crit Care Med. 2020;48(5):709–716. doi: 10.1097/CCM.0000000000004283. [DOI] [PubMed] [Google Scholar]
- 59.Erdahl K. Hotline helping hospitals with ICU capacity is still ringing, but hope is emerging. December 7, 2020. https://www.kare11.com/article/news/health/coronavirus/hotline-helping-hospitals-with-icu-capacity-is-still-ringing-but-hope-is-emerging/89-d79133ce-3753-4ac8-8691-aeb9dcce5d95 Updated December 12, 2020. KARE-TV website.
- 60.Villarroel L., Christ C.M., Smith L., Larsen C. Collaboration on the Arizona surge line: how Covid-19 became the impetus for public, private, and federal hospitals to function as one system. NEJM Catalyst. January 22, 2021 [Google Scholar]
- 61.State of North Dakota State of North Dakota hospital coordination and vulnerable population protection plan 2020 COVID-19 pandemic response. 2020. ND Response website. https://ndresponse.gov/sites/www/files/documents/covid-19/Additional%20Resources/Hospitals%20and%20VP3%20FINAL.pdf
- 62.Wurmb T., Scholtes K., Kolibay F., et al. Hospital preparedness for mass critical care during SARS-CoV-2 pandemic. Crit Care. 2020;24(1):386. doi: 10.1186/s13054-020-03104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hossain T., Ghazipura M., Dichter J.R. Intensive care role in disaster management critical care clinics. Crit Care Clin. 2019;35(4):535–550. doi: 10.1016/j.ccc.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Branson R, Sandrock C, Amundson D, et al. Oxygen conservation strategies during COVID-19 surges. Published May 5, 2021. Accessed May 25, 2021. https://www.chestnet.org/Chestnetorg/Topic-Collections/COVID-19/COVID-in-Focus/Oxygen-Conservation-Strategies-During-COVID-19-Surges
- 65.Minnesota Department of Health Patient care strategies for scarce resource situations. 2021. State of Minnesota website. https://www.health.state.mn.us/communities/ep/surge/crisis/standards.pdf
- 66.Assistant Secretary for Preparedness and Response, United States Department of Health and Human Services. Mass critical care: system level surge capacity: crucial strategies Project ECHO clinical rounds. January 26, 2021. Accessed October 31, 2021. https://www.youtube.com/watch?v=b19Cy2UkUYM
- 67.Michelson K.A., Rees C.A., Sarathy J., et al. Inter-region transfers for pandemic surges. Clin Infect Dis. 2020:ciaa1549. doi: 10.1093/cid/ciaa1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arthur K.R., Kelz R.R., Mills A.M., et al. Interhospital transfer: an independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013;79(9):909–913. doi: 10.1177/000313481307900929. [DOI] [PubMed] [Google Scholar]
- 69.Lyphout C., Bergs J., Stockman W., et al. Patient safety incidents during interhospital transport of patients: a prospective analysis. Int Emerg Nurs. 2018;36:22–26. doi: 10.1016/j.ienj.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 70.Malpass H.C., Enfield K.B., Keim-Malpass J., Verghese G.M. The interhospital medical intensive care unit transfer instrument facilitates early implementation of critical therapies and is associated with fewer emergent procedures upon arrival. J Intensive Care Med. 2015;30(6):351–357. doi: 10.1177/0885066614521964. [DOI] [PubMed] [Google Scholar]
- 71.Flabouris A., Hart G.K., George C. Outcomes of patients admitted to tertiary intensive care units after interhospital transfer: comparison with patients admitted from emergency departments. Crit Care Resusc. 2008;10(2):97–105. [PubMed] [Google Scholar]
- 72.Toner E, Mukherjee V, Hanfling D, et al. Crisis Standards of Care: Lessons from New York City Hospitals’ COVID-19 Experience. A Meeting Report. Baltimore, MD: Johns Hopkins Center for Health Security; 2020.
- 73.Assistant Secretary for Preparedness and Response, United States Department of Health and Human Services Mass critical care: communication and collaboration Project ECHO clinical rounds February 9, 2021. https://www.youtube.com/watch?v=s8n_wdn_lx4
- 74.King M.A., Niven A.S., Beninati W., et al. Evacuation of the ICU: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4 suppl):e44S–e60S. doi: 10.1378/chest.14-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vives R. In Imperial County, medical air teams work around the clock to move COVID-19 patients. Los Angeles Times. July 17, 2020 https://www.latimes.com/california/story/2020-07-17/imperial-county-medical-air-teams-work-around-clock-to-move-covid-19-patients Accessed May 25, 2021. [Google Scholar]
- 76.Haskins P. Carilion Clinic Transfer and Communications Center helps improve ED patient flow, outcomes. ACEP Now. September 19, 2017 http://www.acepnow.com/article/carilion-clinic-transfer-communications-center-helps-improve-ed-patient-flow-outcomes Accessed April 27, 2021. [Google Scholar]
- 77.Kane E.M., Scheulen J.J., Puttgen A., et al. Use of systems engineering to design a hospital command center. Jt Comm J Qual Patient Saf. 2019;45(5):370–379. doi: 10.1016/j.jcjq.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 78.Collins B.E. Use of high-reliability principles in the evolution of a hospital command centre. Healthc Q. 2021;23(4):46–52. doi: 10.12927/hcq.2020.26393. [DOI] [PubMed] [Google Scholar]
- 79.Merkel M.J., Edwards R., Ness J., et al. Statewide real-time tracking of beds and ventilators during coronavirus disease 2019 and beyond. Crit Care Explor. 2020;2(6) doi: 10.1097/CCE.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simmons S., Baum S. The positive impact of establishing an internal transfer center. Nursing Leader. 2021;19(1):33–39. [Google Scholar]
- 81.Dichter J.R., Kanter R.K., Dries D., et al. System-level planning, coordination, and communication: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4 suppl):e87S–e102S. doi: 10.1378/chest.14-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Persoff J., Patel H., Singh S., et al. Expanding the hospital incident command system with a physician-centric role during a pandemic: The role of the physician clinical support supervisor. Journal of Hospital Administration. 2020;9(3):7–10. [Google Scholar]
- 83.Tevis SE, Patel H, Singh S, et al. Impact of a physician clinical support supervisor in supporting patients and families, staff, and the health-care system during the COVID-19 pandemic [published online ahead of print September 10, 2020].Disaster Med Public Health Prep. https://doi.org/10.1017/dmp.2020.345. [DOI] [PMC free article] [PubMed]
- 84.Chandra S., Hertz C., Khurana H., Doerfler M.E. Collaboration between tele-ICU programs has the potential to rapidly increase the availability of critical care physicians—our experience was during coronavirus disease 2019 nomenclature. Crit Care Explor. 2021;3(3):e0363. doi: 10.1097/CCE.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krouss M., Allison M.G., Rios S., et al. Rapid implementation of telecritical care support during a pandemic: lessons learned during the coronavirus disease 2020 surge in New York City. Crit Care Explor. 2020;2(11):e0271. doi: 10.1097/CCE.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carenzo L., Costantini E., Greco M., et al. Hospital surge capacity in a tertiary emergency referral centre during the COVID-19 outbreak in Italy. Anaesthesia. 2020;75(7):928–934. doi: 10.1111/anae.15072. [DOI] [PubMed] [Google Scholar]
- 87.Singh J., Green M.B., Lindblom S., Reif M.S., Thakkar N.P., Papali A. Telecritical care clinical and operational strategies in response to COVID-19. Telemed J E Health. 2021;27(3):261–268. doi: 10.1089/tmj.2020.0186. [DOI] [PubMed] [Google Scholar]
- 88.Borgen I., Romney M.C., Redwood N., et al. From hospital to home: an intensive transitional care management intervention for patients with COVID-19. Popul Health Manag. 2021;24(1):27–34. doi: 10.1089/pop.2020.0178. [DOI] [PubMed] [Google Scholar]
- 89.Andrist E., Clarke R.G., Harding M. Paved with good intentions: hospital visitation restrictions in the age of coronavirus disease 2019. Pediatr Crit Care Med. 2020;21(10):e924–e926. doi: 10.1097/PCC.0000000000002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kandori K., Okada Y., Ishii W., Maebayashi Y., Lizuka R. Association between visitation restriction during the COVID-19 pandemic and delirium incidence among emergency admission patients: a single-center retrospective observational cohort study in Japan. J Intensive Care. 2020;8(90) doi: 10.1186/s40560-020-00511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.