Figure 4.
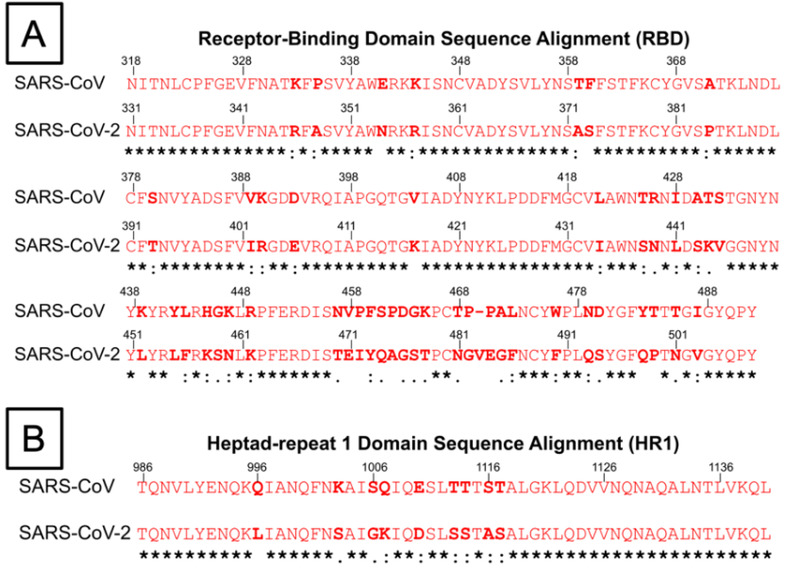
Amino acid sequence alignment of the receptor‐binding domain and heptad repeat 1 (HR1) domain of both SARS‐CoV and SARS‐CoV‐2 virus. Conserved residues between both viruses are marked with asterisks (*), residues with similar properties are marked with a colon (:), while residues with only marginally similar properties are marked with a period (.). A) Amino acid sequence alignment of RBD of SARS‐CoV and SARS‐CoV‐2. The several residue changes in the SARS‐CoV‐2 RBD in comparison to SARS‐CoV allow for higher binding affinity between RBD and ACE‐2 at the RBD–ACE‐2 interface. B) Amino acid sequence alignment of HR1 domains of SARS‐CoV and SARS‐CoV‐2. The residue changes marked within the HR1 domain prompt study into differences in the interactions between HR1 and HR2 domains, which affect 6‐HB formation.
