Abstract
We estimated the seroprevalence of anti‐severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) antibodies in residents of African countries and explored its associated factors. We searched PubMed, EMBASE, PsycINFO, AMED, CINAHL, DOAJ and Google Scholar databases for peer reviewed articles and pre‐prints that reported anti‐SARS‐CoV‐2 antibody seroprevalence of general or specific human populations resident in Africa. The eligible studies were evaluated using Joana Briggs Institute prevalence critical appraisal tool. Twenty‐three studies involving 27,735 individuals were included in our paper. The pooled seroprevalence of anti‐SARS‐CoV‐2 antibodies in Africa was 22% (95%CI: 14–31) with very high heterogeneity (I 2 = 100%, p < 0.001). Seroprevalence was highest in studies conducted in Central Africa compared to Southern Africa, West Africa, North Africa and East Africa respectively. The number of days between the first reported coronavirus disease 2019 case in each country and when a seroprevalence study was conducted was a significant moderator of seroprevalence. Seropositivity was numerically influenced by gender and age of the participants with males and those aged below 50 years being most affected with SARS‐CoV‐2 infection. The highest pooled seroprevalence in Africa reported in this review should be interpreted cautiously due to high heterogeneity between studies. Continued seroprevalence surveillance is warranted to establish Africa's transition towards herd immunity.
Keywords: Africa, antibody, Covid‐19, SARS‐CoV‐2, seroprevalence
Abbreviations
- AMED
AMED Allied and Complementary Medicine
- CINAHL
Cumulative Index of Nursing and Allied Health Literature
- Covid‐19
coronavirus disease 19
- DOAJ
The Directory of Open Access Journals
- EMBASE
Excerpta Medica database
- JBI
Joana Briggs Institute
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- PROSPERO
The International Prospective Register of Systematic Reviews
- PsycINFO
Psychological Information Database
- WHO
World Health Organization
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and its concomitant disease coronavirus disease 2019 (Covid‐19) has been declared a pandemic. 1 Currently, over 122 million Covid‐19 positive cases and over 2.7 million deaths have been reported globally representing a case fatality rate of 2.2%. 2 The detection of SARS‐CoV‐2 in nasopharyngeal swabs using real time‐polymerase chain reaction (qRT‐PCR) has been recommended by WHO as a gold standard method for ascertaining positivity in symptomatic patients. 3 However, the method fails to provide the extent of the population or community exposure to SARS‐CoV‐2 infection. 4 This underestimation of the extent of exposure of the population to SARS‐CoV‐2 by RT‐PCR has been reported by several studies 5 , 6 , 7 and has greatly affected implementation and uptake of infection control and prevention strategies. 8 Serological tests that detect IgG and/or IgM serum antibodies through enzyme‐linked immunosorbent assays (ELISAs), chemilumiscence immunoassays and lateral flow immunoassays (LFIAs) remain the only plausible platforms for providing the collective population exposure to SARS‐CoV‐2 infection. 5 , 9 , 10
Globally, population based and group targeted seroprevalence studies have been conducted with a reported seroprevalence ranging from 0.08% to 31.5% at 95% confidence interval (CI). 4 , 8 However, Africa's anti‐SARS‐CoV‐2 antibodies seroprevalence has been hugely under‐represented as few countries were included (1–3). Seroprevalence studies have the ability to establish the number of people who have at any time been infected. Recently, studies have shown that the rate of reinfection is very low as immunity is able to protect the already infected individuals against repeat SARS‐CoV‐2 infections. 11 , 12 As the pandemic progresses, with efforts being tailored to mass vaccination of the global population including Africa, it is imperative and crucial that a well‐focused and quick estimate of the anti‐SARS‐CoV‐2 seroprevalence be undertaken in Africa. Africa generally has done fewer RT‐PCR tests per population denominator due to resource constraints and hence the true number of infected individuals in Africa is not known. This systematic review and meta‐analysis therefore sought to estimate the current anti‐SARS‐CoV‐2 antibody seroprevalence in Africa.
2. METHODOLOGY
2.1. Search strategy and selection criteria
The study utilized the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines (Figure 1). The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO), registration number CRD42020220074. 13 Systematic literature search was carried out in the following databases: PubMed, EMBASE, PsycINFO, AMED, CINAHL, DOAJ and Google Scholar between December 2020 and April 2021 using the following terms: Covid‐19 OR Covid‐19 OR Covid OR corona virus OR SARS‐CoV‐2 OR SARSCov2 OR SAR‐CoV* OR ncov2 OR nCOV OR 2019‐nCoV or 2019‐nCov or ‘2019 coronavirus’ or ‘2019 corona virus’ OR ‘novel corona virus’ or ‘new corona virus’ or ‘nouveau corona virus’, AND antibody OR anti‐SARS‐CoV‐2 antibody OR SARS‐CoV‐2 IgG OR population OR Seroprevalence OR Sero‐surveillance OR incidence OR extent OR magnitude AND Africa. Efforts were undertaken to identify additional published data through manual hand searching of reference lists from articles that met the inclusion criteria. All the gathered articles were imported into EndNote X9 software (Thompson and Reuters) and duplicates were removed. Article titles, abstracts and full text were independently reviewed by SR, MROC, PK, BCM and SEM for eligibility for inclusion. Any discrepancies were resolved through discussions with MC, ON, BN & EC. The reviewed articles included peer reviewed published articles, and preprints that reported anti‐SARS‐CoV‐2 seroprevalence of the general population, or specific working group domiciled in a specific state/city/region/district of a country within the African continent. The articles were excluded if they reported SARS‐CoV‐2 seroprevalence of non‐African countries or in animal experiments and were not written in English language. Case reports or studies, commentaries, perspectives, editorials, reviews and systematic reviews were also excluded.
FIGURE 1.
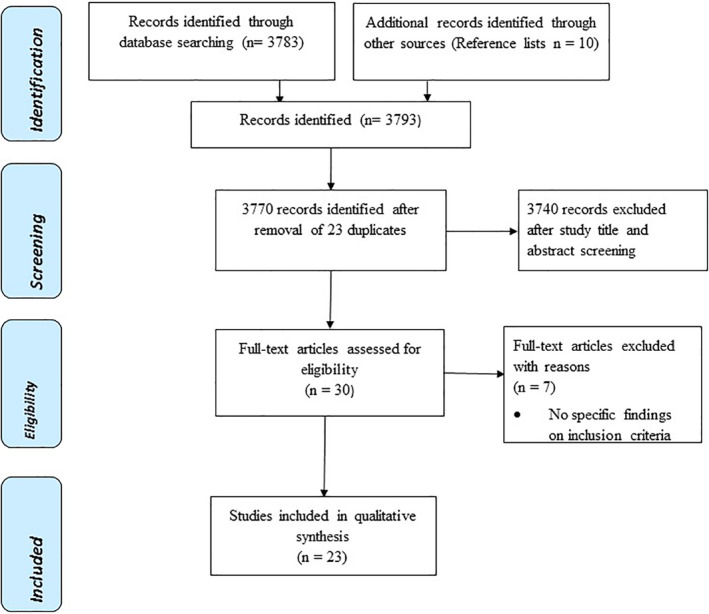
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram
2.2. Data extraction and quality assurance
Data were extracted from articles that met the eligibility criteria as per the adapted and modified Table S1 which was developed in Microsoft Excel 2016. A data extraction table itemized with the name of the author, country, study period, sample selection methods, participants, age range in years, sample size, biological sample used, serological test method, seroprevalence, sensitivity and specificity of serological method was used to collect data (Table S1). Data collation and evaluation from eligible studies was performed by SR, MROC, PK, BCM and SEM. The quality of the eligible studies was assessed using Joana Briggs Institute (JBI) prevalence critical appraisal tool. 14 The individual articles were appraised with a ‘yes’ or ‘no’, ‘unclear’ or ‘not’ ‘applicable’ for their appropriateness of sample frame, sampling technique, sample size, study setting, validity of the immunoassay used, adjustment for non‐response and sound statistical analysis. The number of ‘yes' answers for each individual study across the nine checklist selection criteria was counted and used for the overall inclusion of the study to reduce the risk of study bias. The higher the number of ‘yes’ answers, the higher the chance of inclusion in the review. Thus, using the JBI scale, the scores of 8–9, 5–7 and ≤4 indicated good quality, moderate quality and poor quality studies, respectively. 15 Where there were discrepancies in rating, it was resolved through discussion with all authors (MROC, SR, SEM, AK, PK, MC, ON, BN, EC, & BCM).
2.3. Summary of outcomes and statistical analysis
Meta‐analysis was performed by SEM and AK. The primary outcome of the meta‐analysis was the seroprevalence of anti‐SARS‐CoV‐2 antibodies in African countries. We calculated the weighted‐pooled seroprevalence with a random effects model and assessed heterogeneity with the Hedges Q test and I 2 statistic. The statistical significance for Hedges Q statistic was set at p < 0.1 and I 2 values of greater than 75% indicated a higher heterogeneity between studies. 16 The sample size, sensitivity, specificity, study region in Africa, number of days between the first Covid‐19 reported case and the study period within a specific country, quality of the included studies and publication status were regarded as predetermined sources of heterogeneity and this was explored in subgroup and meta‐regression analyses. The robustness and conclusiveness of the results was assessed using Jackknife sensitivity analysis which omits one study at a time to determine the influence of each study on the overall prevalence. The risk of publication bias and small study effect were detected through assessing the symmetry of a funnel plot, and its significance was assessed using the Egger's test. A non‐significant Egger's test and symmetrical funnel plot indicated low possibility of publication bias. Therefore, p < 0.05 for Egger's test indicated publication bias. The meta‐analysis was not performed for demographic characteristics associated with seroprevalence as the data was not enough and varied across different studies. The statistical analysis was conducted in STATA version 15.1 using the (metareg, metafunnel, metaprop, metaninf, metabias commands) and R‐software version 4.0.5 (meta and metaphor packages).
3. RESULTS
The search strategy identified 3783 articles, and 10 additional articles were identified through review of reference lists. After screening 23 duplicates, 3740 and seven full text articles were successfully excluded; Figure 1 provides the reasons for exclusion. Accordingly, the meta‐analysis of our study included 23 datasets from 23 unique studies. Of these, three were from Nigeria. 17 , 18 , 19 Two were conducted in each of the following countries: Republic of South Africa, 20 , 21 Ethiopia, 22 , 23 Libya 24 , 25 Kenya 26 and Democratic Republic of Congo (DRC). 27 One study was conducted in each of the following countries: Malawi, 28 Togo, 29 Ivory Coast, 30 Zambia, 31 Egypt, 32 Gabon, 33 Congo Brazaville, 34 South Sudan, 35 Cameroon 36 , 37 and Guinea Bissau. 38
The study participants in 10 studies were recruited from the general population, 18 , 22 , 23 , 24 , 31 , 32 , 33 , 34 , 35 , 36 four studies were from health workers, 17 , 27 , 28 , 38 one study combined health workers and community, 25 one study combined health workers, air transport, police and drivers, 29 three studies recruited blood donors, 19 , 20 , 39 one study recruited water front workers, 21 one study involved travelers, 40 one study recruited drivers and their assistants, 26 while the other one recruited gold miners. 30 All studies were conducted in the period between April 2020 and April 2021 except one study by Olayanju et al. (2021) 17 in Nigeria which did not indicate the time/period of the study.
The overall quality of the studies as assessed using the JBI appraisal tool was high based on the rating system, with 74% classified as high quality, 22% moderate quality and 4% low quality (Table S2, Supplementary file).
In terms of biological samples, 15 studies used whole blood, while eight studies used serum samples (Table S1, Supplementary file). The commonly used type of serological test was LFIA, which was performed in nine studies, followed by ELISA in eight studies (Table S1, Supplementary file). Two studies used both the ELISA and LFIA tests, while one study used MN. The largest sample size was 4858 while the least was 98 (Table S1, Supplementary file). The highest anti‐SARS‐CoV‐2 antibody seroprevalence was 63% 20 in the Republic of South Africa (RSA), while the lowest anti‐SARS‐CoV‐2 seroprevalence recorded was 0% 24 in Libya (Figure 2). The sensitivity of test kits reported in this review ranged from 71.1/61.7% to 100.00%, while the specificity ranged from 85.02% to 100.00% (Table S1, Supplementary file) and were within acceptable ranges. 41 However, two studies 32 , 35 did not report on sensitivity. Two studies 31 , 32 did not report on specificity. Twenty studies indicated that their tests were validated, thus two were not validated and one was not indicated (Table S1, Supplementary file). The sensitivity, specificity and the validation results from the included studies showed that the reported prevalence could be relied upon.
FIGURE 2.

Forest plot of the seroprevalence of anti‐severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) antibodies with corresponding 95% confidence intervals in African countries. The proportional weight assigned to a study is shown by the size of the box and the horizontal lines symbolize the 95% confidence intervals (CIs) according to the random effects analysis
The anti‐SARS‐CoV‐2 seroprevalence varied numerically across some demographic characteristics. Six articles reported that seroprevalence was higher among males than females while 12 articles showed that the prevalence was higher in females than males (Table S3, Supplementary file). For those studies that reported age among the Covid‐19 positive cases, the highest seroprevalence was among those who were 50 years or less. For the studies that were conducted in the general population (Table S1, Supplementary file), health care workers (HCW) were not the top most affected group, except in a study by Benn et al. (2021) in which more medical staff and laboratory technicians were infected than other staff (Table S3). Those living in urban settings were numerically more affected (27.82%) than in rural setting (21.41%), in a study by Majiya et al. (2020) in Nigeria. 18 A study by Shaw et al. (2021) in RSA found that seroprevalence was higher in participants with hypertension (15 [15.6%]) than diabetes (10 [10.4%]). 21 , 42 Alemu et al. (2020), in Ethiopia found that prevalence was higher in married 13 (9.8%), compared to single 9 (6.8%), or divorced/widowed 1 (2.8%). 22 Additionally, Alemu et al. (2020) also reported higher seroprevalence of 11 (5.7%) among those with high school and above education compared to those without formal education 1 (6.3%), or primary education (1–8).
A random effects model was utilized to estimate the pooled Anti‐SARS‐CoV‐2 antibody seroprevalence since the heterogeneity between studies was very high (I 2 = 100%, p < 0.001). The overall estimated pooled seroprevalence of anti‐SARS‐CoV‐2 antibodies in Africa involving 27,735 individuals was 22% (95%CI: 14–31; Figure 2). Between African countries the seroprevalence ranged from 0% to 63% (Figure 3)
FIGURE 3.
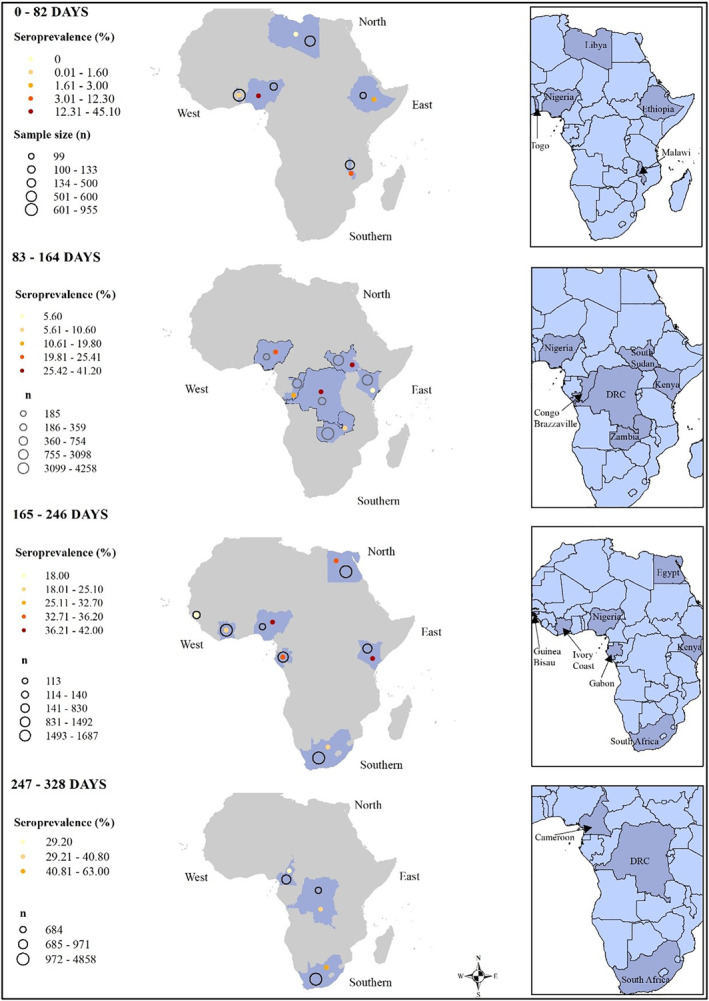
Estimated seroprevalence rates of anti‐severe acute respiratory syndrome coronavirus 2 antibodies in African countries using the Arc Geographical Information System. Number of days represent days since the first reported coronavirus disease 2019 case in each study area
Based on subgroup analysis with respect to study region, seroprevalence of anti‐SARS‐CoV‐2 antibodies was higher in studies conducted in Central Africa (41%, CI: 14–72) compared to Southern Africa (34%, CI: 13–59), West Africa (25%, CI: 13–39), North Africa (13%, CI: 2–32) and East Africa (12%, CI: 2–28; Figure 4). Furthermore, there was a higher seroprevalence of anti‐SARS‐CoV‐2 antibodies in blood donors (33%, CI: 12–60) compared with health care workers (28%, CI: 10–50) and the general population (18%, CI: 8–30; Figure S7, Supplementary File). The seroprevalence of non‐peer reviewed studies (Pre‐prints) was 28% (95%CI: 16–41) higher than that of peer reviewed published studies which was 18% (Figure S8, Supplementary File). Additionally, the seroprevalence was higher for studies of high quality (25%, 95%CI: 17–35) followed by moderate quality (16%, 95%CI: 4–34) and the least were low quality studies (3%, 95%CI: 0–34; Figure S9, Supplementary File). The increase in anti‐SARS‐CoV‐2 antibody seroprevalence was independent of study sample size, sensitivity and specificity of serological tests and their combined interaction effect (Table S7, Supplementary File). Similarly, study quality did not influence the overall seroprevalence (Table S9, Supplementary File). The number of days between the first reported Covid‐19 case in each country and when a seroprevalence study was conducted was a significant moderator of seroprevalence including in its multivariable interaction effect (Tables S8 and S10, Supplementary File).
FIGURE 4.
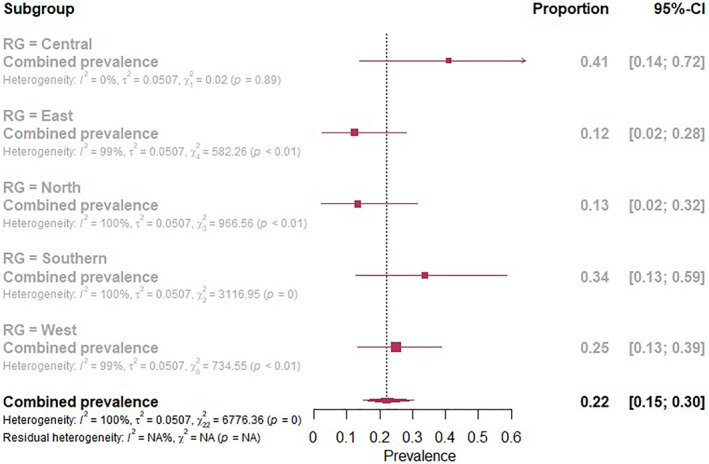
Subgroup analysis of the regions of Africa and anti‐severe acute respiratory syndrome coronavirus 2 antibodies seroprevalence in Africa
The meta‐influence of each study on overall seroprevalence based on Jackknife sensitivity analysis revealed that omitting a high quality study had a proportionate effect on overall anti‐SARS‐CoV‐2 antibody seroprevalence than a low quality study with a range from 18.04 (CI −96.13 to 132.22) to 30.34 (CI: −88.72 to 149.41; Table S15, Supplementary File). The meta‐bias of number of days between the first reported Covid‐19 case in each country and when a seroprevalence study was conducted on overall seroprevalence based on Egger's test and asymmetrical funnel plot was significant (p < 0.001; Table S14, Supplementary File). Nevertheless, the study sample size, sensitivity and specificity of serological tests had no influence on overall anti‐SARS‐CoV‐2 seroprevalence based on Egger's test and symmetrical funnel plots (p > 0.05; Tables S11–S13, Figures S4–S6, Supplementary File).
4. DISCUSSION
Covid‐19 is still the number one public health concern globally. This study provides a comprehensive appraisal of SARS‐CoV‐2 antibody seroprevalence in the human population from the African continent perspective from the available literature (up to 30th April 2021). This was necessitated upon observing that most globally published reviews on this topic critically under represented the Africa's Covid‐19 burden. 4 , 8 , 43 , 44
Overall seroprevalence varied markedly among and between countries (4–8), socio‐demographic and health related characteristics. This review established that the estimated pooled seroprevalence of SARS‐CoV‐2 antibodies in Africa is 22% (95%CI: 14–31) with a seroprevalence range of 0% to 63% between countries which is higher than all global reviews estimates. 4 , 8 , 43 , 44 However, the findings from this review are relatively low as compared to some studies conducted in India. 45 , 46 , 47 Nevertheless, this could mean that even though Africa has experienced lower case morbidities and mortalities as compared to other continents, transmission of Covid‐19 locally is very high.
Based on African region subgroup analysis, seroprevalence of anti‐SARS‐CoV‐2 antibodies was higher in studies conducted in Central Africa (41%) compared to Southern Africa (34%), West Africa (25%), North Africa (13%) and East Africa (12%). As we have shown above, the main factor determining the high seroprevalence is likely the time point at which the study sample was taken, but there are many other factors on the African continent which might contribute to this heterogeneity significantly such as chance, cultural practices, political decision‐making, policies, mitigation efforts, health infrastructure and prevention/control measures and/or the effectiveness of the implementation of such measures as well as occupations. 4 , 44 , 48 , 49 Contrary to many study findings, based on subgroup analysis of participants included in our review, seroprevalence of anti‐SARS‐CoV‐2 antibodies was higher in blood donors followed by health care workers and general population. This could possibly be due to the usage of personal protective equipment and engagements in economic activities. Furthermore, blood donors are generally a healthy subset of the general population hence being the most socio‐economically active group and likely more vulnerable to contract SARS‐CoV‐2.
As expected, the most significant factor affecting the seroprevalence was the number of days between the first reported Covid‐19 case in each country and when a seroprevalence study was conducted and was a significant moderator of seroprevalence including in its multivariable interaction effect with those being conducted earlier showing low level of prevalence.
Surprisingly, sample size, study quality, sensitivity and specificity of serological tests and their combined interaction effect did not influence the overall seroprevalence. This therefore shows that when evaluating the seroprevalence of anti‐SARS‐CoV‐2 antibodies, it is imperative to always consider the effect of length of pandemic exposure period.
A numerically higher seroprevalence was registered in females, while some studies found high seroprevalence among males. 8 , 17 , 18 , 29 , 30 , 39 However, a pooled analysis of systematic and meta‐analysis studies conducted with a global data representation, established that the difference is not statistically significant. 4 , 8 , 43 , 44 The insignificant differences in gender is also substantiated by the absence of gender factor in WHO and African CDC reports and strategic planning agenda. 50 , 51
High anti‐SARS‐CoV‐2 antibody seroprevalence was reported among those aged 50 years or less. 17 , 22 , 26 , 27 , 30 , 33 , 36 , 39 Five of these studies 17 , 20 , 27 , 30 , 39 showed high anti‐SARS‐CoV‐2 antibody seroprevalence among participants with ages between 30 and 50 years. These findings are in accord with a global review with over 58 datasets where they reported high pooled seroprevalence of anti‐SARS‐CoV‐2 among people with age range of 20 to 49 years. Accordingly, studies 52 , 53 , 54 , 55 , 56 , 57 which focused on the characterization of Covid‐19 admitted cases have indicated that most of the patients had ages over 50 years with those critically ill having mean ages of over 60 years and majority of deaths observed among those aged over 65 years. 58 On the other hand, studies have also shown a low prevalence of Covid‐19 among children. 8 , 44 It is more likely that the high involvement in economic activities among individuals of the age group of 30–50 years could have contributed to high seroprevalence rate observed. 44 , 49 However, some studies have reported lack of adherence to Covid‐19 preventive measures among this age group. 59 , 60 , 61 Hence an increase in the enforcement of restrictions on movements, assembly or gathering of people have been strongly advocated in most settings around the world. 61 Much as it is imperative to continue advocating for the prohibitions of movement, gathering, social interactions and the rest of measures, the Covid‐19 battle is multifaceted, demanding well thoughtful strategies on how to practically win the trust of the population and support them to cope with these stringent measures. 62
Similar to other studies, 4 , 63 in this review, settlements, 18 , 45 , 46 , 64 comorbidities, 21 marital status 22 and level of education 22 were associated with rate of anti‐SARS‐CoV‐2 seroprevalence. Particularly, studies from India have reported very high Anti SARS CoV‐2 antibody seroprevalence among the residents of slums. 45 , 46 Practically Covid‐19 has shown and proven to have a gross and multifaceted dynamics on pathogenesis, transmission as well as preventions hence these factors may have an influence in one way or another. 4 , 65 , 66 , 67
Our study calls for more comprehensive SARS‐CoV‐2 seroprevalence studies in African continent to monitor progressive changes in their respective countries. In the context of epidemics and pandemics, such studies might be conducted regularly to allow authorities to assess the spread of the virus and exposure levels of populations. 4 As most African countries are adopting and procuring vaccines which is likely to be in insufficient quantities for everyone in the shortest time period, a plan is still required to monitor SARS‐CoV‐2 seroprevalence to continue assisting in prevention and control efforts. Furthermore, with the emergent evidence that the rate of reinfection of SARS‐CoV‐2 is very low, 11 , 12 we speculate that other countries may decide to exempt the confirmed anti‐SARS‐CoV‐2 antibody seropositive people as a cost cutting measure of vaccine administration.
As the present study represents an African first ‘snapshot’ of SARS‐CoV‐2 seroprevalence based on evaluation of published information, it has a number of limitations. First, after 12 months since the Covid‐19 pandemic started, there is a critical lack of peer‐reviewed, population‐based studies from many countries across the African continent and some studies included here lacked data on most important basic epidemiological variables like sex and age of subjects tested. Furthermore, the disease onset for each country is different hence posing a critical challenge on generalization. We are optimistic that these challenges will be addressed in the coming studies, so that future longitudinal investigations will provide better representative estimates of seroprevalence. This will ensure that conclusive decisions might be reached regarding endemic stability and instability in particular countries.
5. CONCLUSION
Overall, anti‐SARS‐CoV‐2 seroprevalence varied numerically among countries, socio‐demographic and health related characteristics. This review has registered one of the highest pooled seroprevalence (22%) of anti‐SARS‐CoV‐2 antibodies. However, due to inconsistency in the captured data, lack of comprehensive reporting, and methodological flaws, the existing findings should be interpreted with caution. More rigorously executed anti‐SARS‐CoV‐2 seroprevalence studies in Africa are needed to monitor progressive changes in respective countries.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Design of the literature review: Master R.O. Chisale, Sheena Ramazanu, Saul E. Mwale & Balwani C. Mbakaya. Protocol writing and publication in PROSPERO: Master R.O. Chisale, Sheena Ramazanu, Pizga Kumwenda, Obed Nkhata, Mep Chipeta & BCM. Literature search: Master R.O. Chisale, Sheena Ramazanu, Saul E. Mwale & Balwani C. Mbakaya. Data analysis: Master R.O. Chisale, Sheena Ramazanu, Saul E. Mwale, Atipatsa C. Kaminga, Pizga Kumwenda, Mep Chipeta, Obed Nkhata, Billy Nyambalo, Elton Chavura, & Balwani C. Mbakaya. Meta‐analysis: Saul E. Mwale, Atipatsa C. Kaminga. Manuscript writing: Master R.O. Chisale, Sheena Ramazanu, Saul E. Mwale, Pizga Kumwenda, Atipatsa C. Kaminga, Mep Chipeta, Obed Nkhata, Billy Nyambalo, Elton Chavura, & Balwani C. Mbakaya. Critical revision of important intellectual content: Master R.O. Chisale, SR, Saul E. Mwale, Atipatsa C. Kaminga, Pizga Kumwenda, Mep Chipeta, Obed Nkhata, Billy Nyambalo, Elton Chavura, & Balwani C. Mbakaya. All authors have read and approved the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Not applicable. There was no funding for this systematic review; Permission to reproduce materials from other sources; Not applicable. However, where substantial amount of information has been used, due acknowledgement has been made accordingly through citation.
Chisale MRO, Ramazanu S, Mwale SE, et al. Seroprevalence of anti‐SARS‐CoV‐2 antibodies in Africa: a systematic review and meta‐analysis. Rev Med Virol. 2022;32(2):e2271. 10.1002/rmv.2271
DATA AVAILABILITY STATEMENT
We want to confirm that the processed data associated with our results are existing within the systematic review. However, the raw data are available upon request to the Corresponding author (Saul Eric Mwale).
References
REFERENCES
- 1. WHO. WHO declaration of Covid‐19 as global pandemic; 2020. https://www.who.int/news‐room/detail/27‐04‐2020‐who‐timeline‐‐‐Covid‐19
- 2. World Health Organization. WHO weekly epidemiological update on Covid‐19—23 March 2021; 2021. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on‐covid‐19‐‐‐23‐march‐2021. Accessed March 25, 2021.
- 3. Jasper Fuk‐Woo Chan CC‐YY, Kelvin Kai‐Wang To TH‐CT, Wong SC‐Y, et al. Improved molecular diagnosis of COVID‐19 by the novel, highly sensitive and specific Covid‐19‐RdRp/Hel real‐time reverse transcription‐PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58(5):e00310–20. 10.1128/jcm.00310-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rostami A, Sepidarkish M, Lee MMG, et al. SARS‐CoV‐2 seroprevalence worldwide: a systematic review and. Clin Microbiol Infect. 2020;27(October):331–340. 10.1016/j.cmi.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu X, Sun J, Nie S, et al. Seroprevalence of immunoglobulin M and G antibodies against SARS‐CoV‐2 in China. Nat Med. 2020;26(August):1193–1195. 10.1038/s41591-020-0949-6 [DOI] [PubMed] [Google Scholar]
- 6. Posfay‐barbe KM, Andrey DO, Virzi J, et al. Prevalence of immunoglobulin G (IgG) against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and evaluation of a rapid MEDsan IgG test in children seeking medical care. Clin Infect Dis. 2020;72(7):2–5. 10.1093/cid/ciaa1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pollán M, Pérez‐gómez B, Pastor‐barriuso R, et al. Prevalence of SARS‐CoV‐2 in Spain (ENE‐COVID): a nationwide, population‐based seroepidemiological study. Lancet. 2020;396(January);535–544. 10.1016/S0140-6736(20)31483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lai C, Wang J, Hsueh P. Population‐based seroprevalence surveys of anti‐SARS‐CoV‐2 antibody: an up‐to‐date review. Int J Infect Dis. 2020;101:314–322. 10.1016/j.ijid.2020.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Isabel S, Miraglia LG, Gutierrez JM, et al. Evolutionary and structural analyses of SARS‐CoV‐2 D614G spike protein mutation now documented worldwide. Sci Rep. 2020;10:1–9. 10.1038/s41598-020-70827-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Elslande J, Vermeersch P, Vandervoort K, et al. Symptomatic severe acute respiratory syndrome coronavirus 2 (SARS‐ CoV‐2) reinfection by a phylogenetically distinct strain. Clin Infect Dis. 2021;2(Xx Xxxx):1–2. 10.1093/cid/ciaa1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Griffin S. Covid‐19: antibodies protect against reinfection for at least six months, study finds. BMJ. 2020;371:33369366. 10.1136/bmj.m4961 [DOI] [PubMed] [Google Scholar]
- 12. Marsden BD, Cox S, James T, et al. Antibody status and incidence of SARS‐CoV‐2 infection in health care workers. N Engl J Med. 2020:1–8. 10.1056/NEJMoa2034545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mbakaya B, Chisale M, Kumwenda P, et al. COVID‐19 antibody population seroprevalence in Africa: a systematic review; 2020:1–4. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020220074
- 14. Munn Z, Moola S, Lisy K, Riitano DTC. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. 2015;13(3):147‐153. 10.1097/XEB.0000000000000054 [DOI] [PubMed]
- 15. Galanis P, Vraka I, Fragkou D, Bilali AKD, Kaitelidou D. Seroprevalence of SARS‐CoV‐2 antibodies and associated factors in healthcare workers: a systematic review and meta‐analysis. J Hosp Infect. 2021;108:120–134. 10.1016/j.jhin.2020.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thompson SG, Deeks JJAD. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(557e60):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olayanju O, Bamidele O, Edem F, et al. SARS‐CoV‐2 seropositivity in asymptomatic frontline health workers in Ibadan, Nigeria. Am J Trop Med Hyg. 2021;104(1):91–94. 10.4269/ajtmh.20-1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Majiya H, Balogu VT, Musa DA, et al. Seroprevalence of Covid‐19 in Niger State. Preprint. 2020;(June):1–23. 10.1101/2020.08.04.20168112 [DOI] [Google Scholar]
- 19. Ifeorah I. Sero‐pravelence of SARS CoV‐2 IgM and IgG antibodies amongst blood donors in Nigeria. Preprint. 2021:1–13. 10.21203/rs.3.rs-151037/v1 [DOI]
- 20. Sykes W, Mhlanga L, Swanevelder R, et al. Prevalence of anti‐SARS‐CoV‐2 antibodies among blood donors in Northern Cape, KwaZulu‐Natal, Eastern Cape, and Free State Provinces of South Africa in January 2021. Preprint. 2021;(January):1–11. [Google Scholar]
- 21. Alexandra J, Id S, Meiring M, et al. Higher SARS‐CoV‐2 seroprevalence in workers with lower socioeconomic status in Cape. PLoS One. 2021;96:1–12. 10.1371/journal.pone.0247852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nega Alemu B, Addissie A, Mamo G, et al. Sero‐prevalence of anti‐SARS‐CoV‐2 antibodies in Addis Ababa, Ethiopia. Preprint. 2020:1–16. 10.1101/2020.10.13.337287 [DOI] [Google Scholar]
- 23. Kempen JH, Abashawl A, Suga HK, et al. SARS‐CoV‐2 serosurvey in Addis Ababa, Ethiopia. Am J Trop Med Hyg. 2022;103(5):2022–2023. 10.4269/ajtmh.20-0816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abdelghffar AF, Elhassi AF, Elmehdawi R, et al. Population‐based random survey for detection of Covid‐19 infection and seroprevalence in Benghazi‐ Libya may/2020 abstract. J Prev Med. 2020;5(4):1–6. 10.36648/2572-5483.5.4.19 [DOI] [Google Scholar]
- 25. Kammon AM, El‐arabi AA, Erhouma EA, Mehemed TM, Mohamed OA. Seroprevalence of antibodies against SARS‐CoV‐2 among public community and health‐care workers in Alzintan City of Libya. Preprint. 2020:1–8. 10.1101/2020.05.25.20109470 [DOI] [Google Scholar]
- 26. Kagucia EW, Karani A, Gitonga JN, et al. Seroprevalence of anti‐SARS‐CoV‐2 IgG antibodies among truck drivers and assistants in Kenya. Preprint. 2021:1–11. 10.1101/2021.02.12.21251294 [DOI] [Google Scholar]
- 27. Mukwege D, Byabene AK, Akonkwa EM, et al. High SARS‐CoV‐2 seroprevalence in healthcare workers in Bukavu, Eastern Democratic Republic of Congo. Am Soc Trop Med Hyg. 2021;104(4):1526–1530. 10.4269/ajtmh.20-1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chibwana MG, Jere KC, Kamng R, et al. High SARS‐CoV‐2 seroprevalence in health care workers but relatively low numbers of deaths in urban Malawi [version 1; peer review: awaiting peer review]. Wellcome Open Res. 2020:1–8.34632082 [Google Scholar]
- 29. Halatoko WA, Rodion Y, Id K, et al. Prevalence of SARS‐CoV‐2 among high‐risk populations in Lome. PLoS One. 2020;36:1–12. 10.1371/journal.pone.0242124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Milleliri JM, Coulibaly D, Nyobe B, et al. SARS‐CoV‐2 infection in Ivory Coast: a serosurveillance survey among gold mine workers. Preprint. 2021. 10.1101/2021.01.27.21249186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mulenga LB, Hines JZ, Fwoloshi S, et al. Articles Prevalence of SARS‐CoV‐2 in six districts in Zambia in July, 2020: a cross‐sectional cluster sample survey. Lancet Glob Heal. 2021;9(21):1–9. 10.1016/S2214-109X(21)00053-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Id MRG, El AS, Id R, et al. Incidence, household transmission, and neutralizing antibody seroprevalence of Coronavirus Disease 2019 in Egypt: results of a community‐based cohort. PLoS Pathog. 2021:1–13. 10.1371/journal.ppat.1009413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nzoghe AM, Leboueny M, Kamgaing EK, et al. Circulating anti‐SARS‐CoV‐2 nucleocapsid (N)‐protein antibodies and anti‐SARS‐CoV‐2 spike (S)‐protein antibodies in an African setting: herd immunity, not there yet ! BMC Res Notes. 2021;14:3–6. 10.1186/s13104-021-05570-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Batchi‐bouyou AL, Lobaloba L, Ndounga M, et al. International journal of infectious diseases high SARS‐CoV‐2 IgG/IgM seroprevalence in asymptomatic Congolese in Brazzaville, the Republic of Congo. Int J Infect Dis. 2021;106:3–7. 10.1016/j.ijid.2020.12.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wiens KE, Mawien PN, Rumunu J, et al. Seroprevalence of anti‐SARS‐CoV‐2 IgG antibodies in Juba, South Sudan: a population‐based study. Preprint. 2021:1–28. 10.1101/2021.03.08.21253009 [DOI] [Google Scholar]
- 36. Wanda F, Mama L, Orel E, et al. SARS‐CoV‐2 antibody seroprevalence and associated risk factors in an urban district in Cameroon. Preprint. 2021:1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nwosu KD, Fokam J, Wanda F, et al. SARS‐CoV‐2 Antibody Seroprevalence and Associated Risk Factors in an Urban District in Cameroon. Preprint. 2021:1–21. 10.2139/ssrn.3812428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benn CS, Salinha A, Mendes S, et al. SARS‐CoV2 sero‐survey among adults involved in health care and health research in Guinea‐Bissau, West Africa. Preprint. 2021:1–14. 10.1101/2021.03.06.21253046 [DOI] [Google Scholar]
- 39. Uyoga S, Ifedayo M, Adetifa O, Henry K, Karanja JN. Seroprevalence of anti‐SARS‐CoV‐2 IgG antibodies in Kenyan blood donors. Sci Rep. 2020;82(January):79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bianga Katchunga P, Murhula A, Akilimali P, et al. Séroprévalence des anticorps anti SARS‐Cov‐2 parmi les voyageurs et travailleurs dépistés à la clinique Saint Luc de Bukavu, à l´Est de la République Démocratique du Congo, de mai en août 2020. Pan Afr Med J. 2021;38:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. WHO. Antigen‐detection in the diagnosis of SARS‐CoV‐2 infection using rapid immunoassays. Report. 2020;(September):1–9. [Google Scholar]
- 42. Shaw JA, Meiring M, Cummins T, et al. Higher SARS‐CoV‐2 seroprevalence in workers with lower socioeconomic status in Cape Town, South Africa. PLoS ONE. 2021;16(2):e0247852. 10.1371/journal.pone.0247852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Franceschi VB, Santos AS, Barreto A, et al. Population‐based prevalence surveys during the Covid‐19 pandemic: a systematic review. Rev Med Virol. 2020;(November):1–15. 10.1002/rmv.2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen X, Chen Z, Azman AS, et al. Articles serological evidence of human infection with SARS‐CoV‐2: a systematic review and meta‐analysis. Lancet Glob Heal. 2021;9(5):e598–e609. 10.1016/S2214-109X(21)00026-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. George CE, Inbaraj LR, Witte LP. High seroprevalence of Covid‐19 infection in a large slum in South India: what does it tell us about managing a pandemic and beyond? J Infect Dis Epidemiol. 2021;149:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murhekar MV, Bhatnagar T, Selvaraju S, et al. SARS‐CoV‐2 antibody seroprevalence in India, August–September, 2020: findings from the second nationwide household serosurvey. Lancet Glob Health. 2021;9:257–266. 10.1016/S2214-109X(20)30544-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Malani A, Shah D, Kang G, et al. Seroprevalence of SARS‐CoV‐2 in slums versus non‐slums in Mumbai, India. Lancet Glob Heal. 2020;9(2):e110–e111. 10.1016/S2214-109X(20)30467-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH. Correlation between universal BCG vaccination policy and reduced mortality for COVID‐19. 2020. https://www.medrxiv.org/content/10.1101/2020.03.24.20042937v2.full.pdf
- 49. Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD. How will country‐based mitigation measures influence the course of the Covid‐19 epidemic? Lancet. 2020;2019(20):931–934. 10.1016/S0140-6736(20)30567-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. World Health Organization. Strategic response plan for the World Health Oragnisiation African RegionWorld Health Organization (WHO) African regional website; 2020:1–10. https://www.afro.who.int/sites/default/files/2020‐06/SPRPBUDGET0520_01.pdf. Accessed May 15, 2021.
- 51. Africa CDC. Outbreak Brief # 57: coronavirus disease 2019 (Covid‐19) pandemic. CDC Africa; 2021. https://africacdc.org/download/outbreak‐brief‐57‐coronavirus‐disease‐2019‐covid‐19‐pandemic/. Accessed May 15, 2021. [Google Scholar]
- 52. Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with Covid‐19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lacent Haematol. 2020;7:737–745. 10.1016/S2352-3026(20)30251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu W, Tao Z, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chinise Med J. 2020;133(9):1032–1038. 10.1097/CM9.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li L, Huang T, Zheng W, et al. Covid‐19 patients’ clinical characteristics, discharge rate, and fatality rate of meta‐analysis. J Med Virol. 2020;92(April):577–583. 10.1002/jmv.25757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ranzani OT, Bastos LSL, Gelli JGM, et al. Articles Characterisation of the first 250 000 hospital admissions for Covid‐19 in Brazil: a retrospective analysis of nationwide data. Lancent Respir Med. 2021;2600(20):1–418. 10.1016/S2213-2600(20)30560-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Almazeedi S, Al‐youha S, Jamal MH, et al. EClinicalMedicine characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with Covid‐19 in Kuwait. EClinicalMedicine. 2020;24:100448. 10.1016/j.eclinm.2020.100448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nikpouraghdam M, Jalali A, Alishiri G, et al. Epidemiological characteristics of coronavirus disease 2019 (COVID‐19) patients in Iran: a single center study, J Clin Virol. 2020;127;104378. 10.1016/j.jcv.2020.104378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hager E, Odetokun IA, Bolarinwa O, Zainab A, Okechukwu O, Al‐Mustapha AI. Knowledge, attitude, and perceptions towards the 2019 Coronavirus Pandemic: a bi‐national survey in Africa. PLoS One. 2020;15:1–13. 10.1371/journal.pone.0236918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Doherty FV, Odeyemi OA, Adeola A, Amolegbe OAF. Evaluation of knowledge, impacts and government intervention strategies during the Covid‐19 pandemic in Nigeria. Data Br. 2020:1–10. 10.1016/j.dib.2020.106177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mbakaya B, Frank S, Joseph Wu MC. Community‐based interventions for preventing Covid‐19 transmission in low‐ and middle‐income countries: a systematic review. PROSPERO. 2020. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020204984
- 62. Omaka‐amari LN, Aleke CO, Obande‐ogbuinya NE, Ngwakwe PC, Nwankwo O, Afoke EN. Coronavirus (Covid‐19) pandemic in Nigeria: preventive and control challenges within the first two months of outbreak. Afr J Reprod Heal. 2020;2020(June):87–97. 10.29063/ajrh2020/v24i2s.13 [DOI] [PubMed] [Google Scholar]
- 63. Yin T, Li Y, Ying Y, Luo Z. Prevalence of comorbidity in Chinese patients with Covid‐19: systematic review and meta‐analysis of risk factors. BMC Infect Dis. 2021:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Khan SMS, Qurieshi MA, Id IH, et al. Seroprevalence of SARS‐CoV‐2 specific IgG antibodies in District Srinagar, northern India—a cross‐sectional study. PLoS One. 2020;15:e0239303. 10.1371/journal.pone.0239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Centers for Disease Control and Prevention (CDC). Health equity considerations and racial and ethnic minority groups; 2021. https://www.cdc.gov/coronavirus/2019‐ncov/community/health‐equity/race‐ethnicity.html. Accessed March 17, 2021.
- 66. Chhikara BS, Rathi B, Singh J. Corona virus SARS‐CoV‐2 disease Covid‐19: infection, prevention and clinical advances of the prospective chemical drug therapeutics. Chem Biol. 2020;7(1):63–72. [Google Scholar]
- 67. Beheshtkhoo N, Alipour MH, Nemati R, et al. A review of Covid‐19: the main ways of transmission and some prevention solutions, clinical symptoms, more vulnerable human groups, risk factors, diagnosis, and treatment. J Environ Treat Tech. 2020;8(3):884–893. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
We want to confirm that the processed data associated with our results are existing within the systematic review. However, the raw data are available upon request to the Corresponding author (Saul Eric Mwale).


