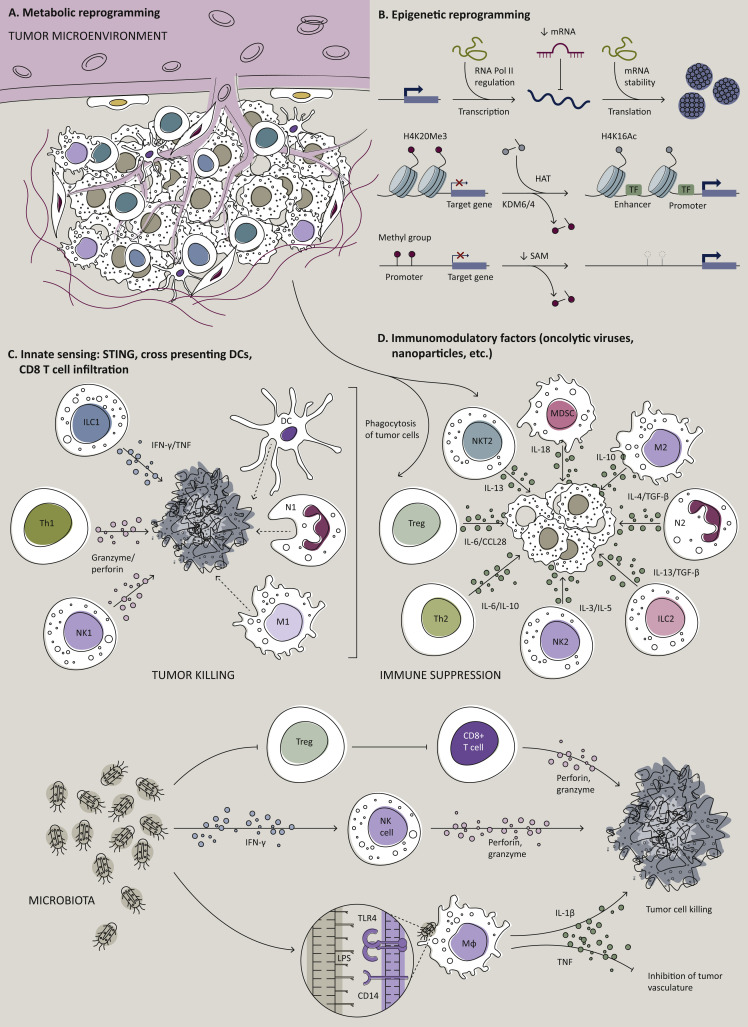
Figure 3.
Potential strategies to heat up the TME.
(A) Targeting cellular metabolism and certain metabolites within the TME to reduce immunosuppressive regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), or to generate metabolically fit T cells with better mitochondrial activity to protect against the tumor. Image captions: °, immune cell; ˆ, stroma cell; ∗, cancer cell. (B) Targeting epigenetic modulators to either promote immunogenicity of tumor cells or to re-educate TAMs, MDSCs, or Tregs for the support of T-cell effector functions.71 (C) Induced activation of the innate immune sensing system with stimulator of STING agonists or boosting cross-presenting DCs to promote tumor Ag-specific T-cell trafficking or function within the TME. (D) Creating an inflamed TME via OVs or nanoparticle delivery of key immunomodulatory factors.71,72 In this regard, OVs deserve a special mention, as they are capable of tumor-specific replication, which can provide a therapeutic opportunity.72 Briefly, OVs are naturally occurring or genetically engineered viral species, able to selectively kill cancer cells without damaging healthy tissue.72 Their mechanism of action is multimodal, as the injection of OVs in primary/accessible tumors induces immunogenic cell death of tumor cells, promoting the build-up of an inflamed TME.71 In fact, OVs support natural killer (NK) cell and T-cell immune responses, ultimately improving the lysis of OV-infected cancer cells. Moreover, the activation of antiviral innate immunity, such as type I IFNs and IFN-stimulated genes, promotes the release of damage- and pathogen-associated molecular patterns, the exposure of viral/tumor Ag, as well as the polarization of TAMs towards antitumor M1 phenotype within the TME.73,74 The consequent OV-mediated upregulation of immune checkpoints (i.e. programmed death-ligand 1 and programmed death-ligand 2, PD-L1 and PD-L2, respectively) provides a rationale for combination immunotherapy of OVs plus ICB. CCL28, chemokine (C–C motif) ligand 28; DC, dendritic cell; HAT, histone acetyltransferase; IFN, interferon; IL, interleukin; ILC1/2, innate lymphoid cells 1/2; KDM, histone lysine demethylase; lncRNA, long non-coding RNA; LPS, lipopolysaccharides; M1, classically activated macrophages; M2, alternatively activated macrophages; MDSC, myeloid-derived suppressor cell; miRNA, microRNA; N1, antitumorigenic neutrophil; N2, pro-tumorigenic neutrophil; NK, natural killer; OV, oncolytic virus; RNA Pol II, RNA polymerase II; SAM, S-adenosyl methionine; STING, stimulator of interferon genes; TAM, tumor-associated macrophage; TF, transcription factor; TGF- β, transforming growth factor-β; Th, T helper cell; TLR, Toll-like receptor; TNF, tumor necrosis factor; Treg, regulatory T cell.