1.
Dear Editor,
1.
Psoriasis is a cutaneous immune‐mediated inflammatory disease resulting from a combination of polygenic and environmental factors. Various endogenous and exogenous triggers have been observed to initiate the onset or exacerbation of the disease via a T‐cell mediated response. 1 We present a case of a patient who experienced exacerbation of psoriasis after receiving the BNT162b2 vaccine against SARS‐CoV‐2.
A 65‐year‐old male with a history of hepatocellular carcinoma previously treated with nivolumab and poorly controlled psoriasis since childhood presented with an exuberant flare that began 1 week after receiving the first dose of the BNT162b2 mRNA COVID‐19 vaccine. Of note, the patient received infusions of nivolumab, a PD‐1 inhibitor, for hepatocellular carcinoma for 9 months, which were discontinued 3 months prior to this psoriatic flare due to an excellent treatment response. Although not ideally controlled in the setting of nivolumab therapy, his psoriasis was stable on apremilast and topical calcipotriene and clobetasol and became less active after cessation of immunotherapy. The patient was previously seen in clinic 1 day prior to his vaccine appointment to discuss better management options for his psoriasis. Physical exam revealed very thin plaques involving about 10% of the body surface area (BSA), which was the patient's baseline. The patient returned 3 weeks later with significantly worse pruritus and scattered plaques that had increased in BSA, erythema, and scale (Figure 1). He reported the symptoms began 1 week after getting the BNT162b2 vaccine and denied any other possible triggers, including changes in medications, discontinuation of his psoriasis treatment, or symptoms of and known exposure to SARS‐CoV‐2. A skin biopsy was not performed as the lesions clinically appeared similar to previous flares. Considering the drastic change in the severity of his psoriasis within a matter of weeks and no other known reportable causes, the acute worsening of his skin disease appeared to be provoked by the BNT162b2 vaccine. The patient received the second vaccine dose and followed up 3 weeks later. He did not experience further aggravation of his flare.
FIGURE 1.
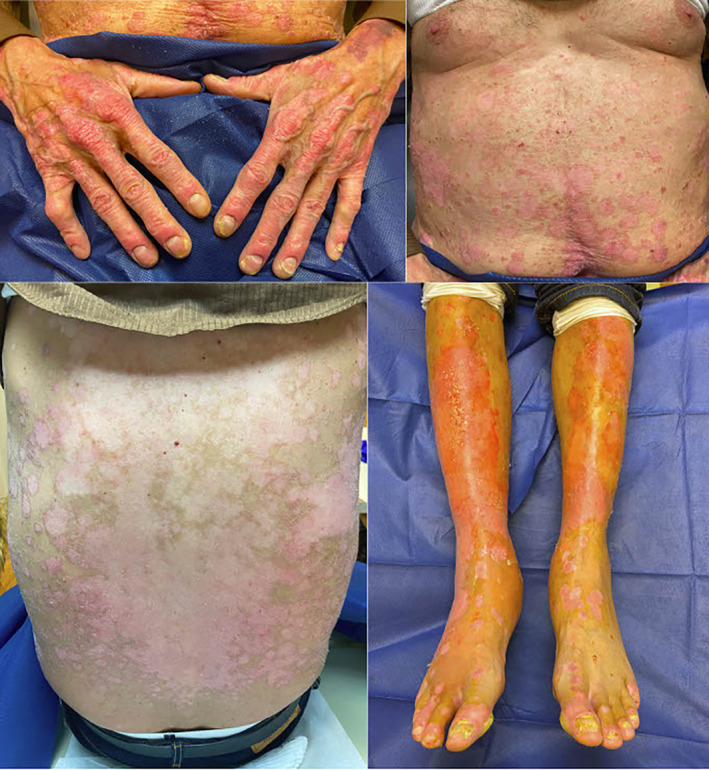
Erythematous scaly plaques throughout the chest, abdomen, back, and extremities
Infections are a known trigger of psoriasis with various morphologies, including plaque psoriasis, guttate psoriasis, and pustular psoriasis, reported following numerous viral and bacterial infections. 1 Furthermore, exacerbations have been observed after various immunizations, including the influenza, Bacillus Calmette‐Guérin (BCG), tetanus‐diphtheria, and pneumococcal polysaccharide vaccines, in settings when other provoking factors were excluded. The mechanisms underlying these responses may rely on induction of interleukin 6 (IL‐6), which stimulates T helper 17 (Th17) cells to produce IL‐22, the cytokine that plays a key role in keratinocyte proliferation characteristic of psoriasis. 2 Additionally, accounts of exacerbations in the plaque, guttate, and pustular forms have been observed days to weeks following infection with SARS‐CoV‐2. 3 , 4 , 5 , 6
Our patient had previously been treated with nivolumab, which is also known to trigger and exacerbate psoriasis; however, this medication was discontinued 3 months prior to this flare with some subsequent improvement in his psoriasis. 7 Furthermore, influenza vaccination during active immunotherapy treatment for cancer appears to result in higher rates of vaccine‐mediated immunologic side effects. 8 Consequently, it is unknown if prior treatment with PD‐1 inhibitor immunotherapy predisposed our patient to a vaccine‐mediated autoimmune disease flare.
Given psoriasis exacerbations have been reported after infections and immunizations, our case demonstrates that flares following the vaccine are possible. Three other cases of psoriasis flares after the COVID‐19 vaccine have been documented in the literature. 9 , 10 Nonetheless, experts strictly recommend COVID‐19 vaccination for psoriasis patients, considering the low incidence of psoriasis diagnoses and flares following various vaccines and the ability to appropriately manage the sequelae. 11 Our patient was at high risk for severe COVID‐19, therefore he proceeded to receive the second dose of the vaccine.
CONFLICT OF INTEREST
There are no conflicts of or competing interests to disclose.
ETHICS STATEMENT
No ethical approval is required for case reports at our institution.
AUTHOR CONTRIBUTIONS
All authors have contributed to the manuscript. Andreas Kaubisch and Beth N. McLellan evaluated the patient in clinic. Karolina Mieczkowska was present for the patient evaluation and photos. Karolina Mieczkowska, Andreas Kaubisch, and Beth N. McLellan wrote and revised the manuscript draft.
INFORMED CONSENT
Verbal consent from the patient to report his case in an international journal was obtained.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sbidian E, Madrange M, Viguier M, et al. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br J Dermatol. 2019;181(6):1304‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gunes AT, Fetil E, Akarsu S, Ozbagcivan O, Babayeva L. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kutlu O, Metin A. A case of exacerbation of psoriasis after oseltamivir and hydroxychloroquine in a patient with COVID‐19: will cases of psoriasis increase after COVID‐19 pandemic? Dermatol Ther. 2020;33(4):e13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozaras R, Berk A, Ucar DH, Duman H, Kaya F, Mutlu H. Covid‐19 and exacerbation of psoriasis. Dermatol Ther. 2020;33(4):e13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gananandan K, Sacks B, Ewing I. Guttate psoriasis secondary to COVID‐19. BMJ Case Rep. 2020;13(8):e237367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shakoei S, Ghanadan A, Hamzelou S. Pustular psoriasis exacerbated by COVID‐19 in a patient with the history of psoriasis. Dermatol Ther. 2020;33(6):e14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Politi A, Angelos D, Mauri D, Zarkavelis G, Pentheroudakis G. A case report of psoriasis flare following immunotherapy: Report of an important entity and literature review. SAGE Open Med Case Rep. 2020;8:2050313X19897707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laubli H, Balmelli C, Kaufmann L, et al. Influenza vaccination of cancer patients during PD‐1 blockade induces serological protection but may raise the risk for immune‐related adverse events. J Immunother Cancer. 2018;6(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: A registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krajewski PK, Matusiak L, Szepietowski JC. Psoriasis flare up associated with second dose of Pfizer‐BioNTech BNT16B2b2 COVID‐19 mRNA vaccine. J Eur Acad Dermatol Venereol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diotallevi F, Campanati A, Radi G, et al. Vaccination against SARS‐CoV‐2 and psoriasis: the three things every dermatologist should know. J Eur Acad Dermatol Venereol. 2021;35(7):e428‐e430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


