Dear Editor,
To date, several individuals have received COVID‐19 vaccinations; therefore, the development of adverse skin reactions is expected. We report a peculiar cutaneous eruption post anti‐Covid‐19 vaccination (BNT162B2/Pfizer) in a woman without comorbidities, with a previous history of Covid 19 infection. A 51‐year‐old female patient, in good general health, had a symptomatic Covid‐19 infection in April 2020 with fever, arthralgia, loss of taste and smell for 3 months and peripheral leg neuropathy that lasted for a few days; at this time, she did not present any skin manifestation. The initial anti‐spike antibody value was 70.2 AU/ml (n.v. AU <12 ml). In early January 2021, 6 h after the first dose anti‐Covid‐19 vaccination (BNT162B2/Pfizer), she presented edema and pain at the injection site and, the next day, arthralgia, fever, and the appearance of an itchy maculopapular rash. Initially, the eruption was localized on the upper limbs (volar surface) (Figure 1A), retro‐auricular region and, 1‐day later, on the trunk. Anti‐spike antibody value at this time was >400 AU/ml and neutralizing antibodies titer was 1:640. Nasal swab proved negative for Sars‐Cov‐2 virus. Two skin biopsies for histological examination and direct immunofluorescence were done. By histology, a lymphocytic vasculitis with lymphocytes infiltrating the wall of small dermal vessels, with endothelial swelling was noticed (Figure 1B) in absence of thrombi. A predominance of T CD4+ lymphocytes (Dako) over T CD8+ cells (Dako) was noticed (Figure 1C,D); immunostaining with anti‐SARS‐CoV‐2 nucleocapsid protein antibody (Sino Biological) did not show any specific reactivity. Direct immunofluorescence directed to IgG, IgM, IgA fibrinogen, and C3 (Dako, Denmark) did not reveal any deposits in the vessels. Further blood analyses to exclude concomitant viral reactivations (in particular HHV‐6‐DNA, HHV‐7‐DNA, EBV‐DNA, and CMV‐DNA) proved negative; parvovirus IgG and IgM were absent. Therapy with systemic antihistamine and local steroid led to the resolution of the manifestations within a week. Due to the high level of immunization demonstrated by the serological test, the second dose of vaccine was not carried out. Vaccination in previously infected subjects is still debated as an opportunity to strengthen the defenses against the virus; however, the possible side effects are not yet fully known. 1 New scientific evidences point to the possibility to perform a single dose vaccination in those who have a history of previous Covid‐19 infection. 2 In particular, a single dose of mRNA vaccine elicits very rapid immune responses in seropositive individuals with post‐vaccine antibody titers comparable to or exceed titers found in naïve individuals who received two vaccinations. 2 By now, the cutaneous patterns of symptomatic disease are well described, and the maculopapular manifestation is one of the most frequently seen and associated with a direct effect of the virus on the skin. 3 After the observation of our single case, it can be hypothesized that the immune response to the virus/vaccination is also involved in the development of skin eruptions, targeting small vessels. New insights about cross‐reactivity between human tissue and SARS‐CoV‐2 have been recently demonstrated and the possibility of development of autoimmune disease reported. 4 , 5 Immune response against the viral antigen following infection or vaccination can react with human tissue because of mimicry. 4 The involvement of the vessel might explain the late vascular side effect such as chilblain eruption. In fact, histologically, the presence of lymphocytic vasculitis has been found also in Covid‐19 chilblain eruption that; however, has in common with our case only the benign clinical course. 6 , 7 , 8 Lymphocytic vasculitis can be find also in lupus erythematosus; however, direct immunofluorescence and autoimmune serology proved negative in our patient. The clinical features of our patient point to a post‐vaccination rash similar to a paraviral eruption, rather than an allergic vaccine rash, which usually presents with anaphylactic or urticarial features. 3 The timing of the eruption favors a post‐vaccine eruption rather than a delayed reaction to SARS‐CoV2 infection. In summary, we describe the occurrence of a peculiar post Covid‐19 vaccination maculopapular rash characterized by lymphocytic vasculitis as the main histological finding, rapidly responding to systemic antihistamine and local steroid therapy.
FIGURE 1.
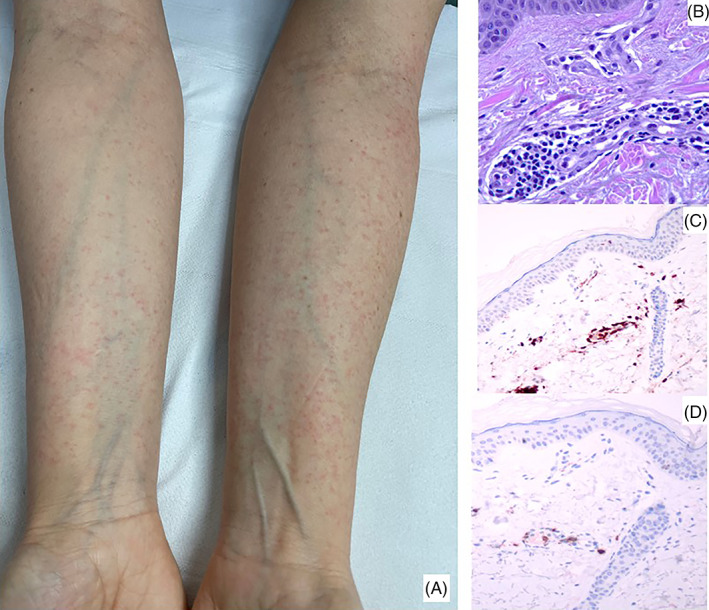
(A) An itchy maculopapular rash localized symmetrically on both arms (B) perivascular lymphocytic infiltrate with endothelial swelling of small vessels (hematoxylin and eosin, x40); (C) CD4+ lymphocytes predominate over CD8+ cells (D) in the perivascular infiltrate (x20)
CONFLICT OF INTEREST
Any conflict of interest disclosures.
AUTHOR CONTRIBUTION
All authors have contributed to the conception and the design of the study. All authors have contributed to the manuscript revision and they all have read and approved the submitted version.
ETHICS STATEMENT
The patient, that is also an author of the manuscript, has given her written informed consent to the publication of her case details.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author, [C.V.]. The data are not publicly available due to containing information that could compromise the privacy of participant.
REFERENCES
- 1. Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID‐19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2020;9(4):1423‐1437. 10.1016/j.jaip.2020.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bradley T, Grundberg E, Selvarangan R. Antibody responses boosted in seropositive healthcare workers after single dose of SARS‐CoV‐2 mRNA vaccine. medRxiv. 2021;5:21251078. 10.1101/2021.02.03.21251078 [DOI] [Google Scholar]
- 3. Marzano AV, Genovese G, Moltrasio C, et al. Italian SkinCovid‐19 network of the Italian Society of Dermatology and Sexually Transmitted Diseases (SIDeMaST). The clinical spectrum of COVID‐19‐associated cutaneous manifestations: an Italian multicentre study of 200 adult patients. J Am Acad Dermatol. 2021;84(5):1356‐1363. 10.1016/j.jaad.2021.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS‐CoV‐2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2021;11:617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gambichler T, Scholl L, Dickel H, Ocker L, Stranzenbach R. Prompt onset of Rowell's syndrome following the first BNT162b2 SARS‐CoV‐2 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e415‐e416. 10.1111/jdv.17225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sohier P, Matar S, Meritet JF, Laurent‐Roussel S, Dupin N, Aractingi S. Histopathologic features of chilblainlike lesions developing in the setting of the coronavirus disease 2019 (COVID‐19) pandemic. Arch Pathol Lab Med. 2021;145(2):137‐144. 10.5858/arpa.2020-0613-SA [DOI] [PubMed] [Google Scholar]
- 7. Colmenero I, Santonja C, Alonso‐Riaño M, et al. SARS‐CoV‐2 endothelial infection causes COVID‐19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183(4):729‐737. 10.1111/bjd.19327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hubiche T, Cardot‐Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon‐alpha response characteristics of patients with chilblain‐like lesions during the COVID‐19 pandemic. JAMA Dermatol. 2021;157(2):202‐206. 10.1001/jamadermatol.2020.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, [C.V.]. The data are not publicly available due to containing information that could compromise the privacy of participant.


