Figure 1.
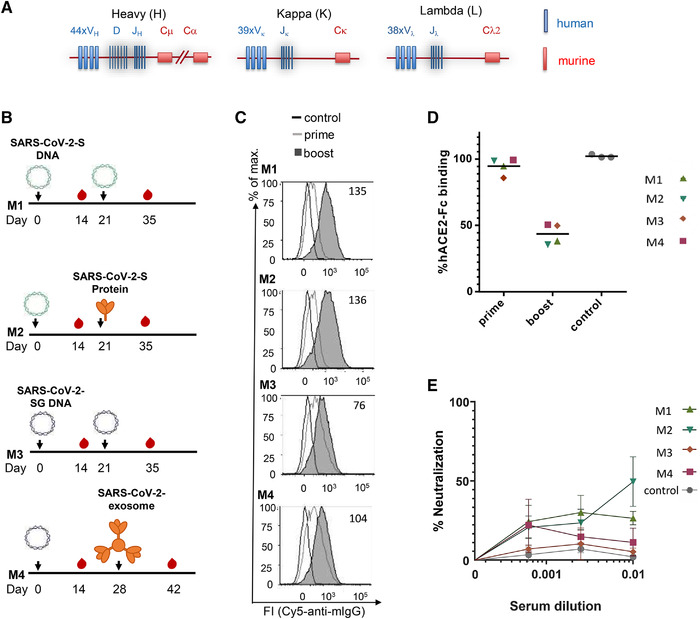
Immunization of TRIANNI mice for induction of SARS‐CoV‐2 neutralizing antibodies. TRIANNI mice harboring the entire human Ig variable region repertoire (A) were primed by intramuscular electroporation with expression plasmids for WT SARS‐CoV‐2‐S (M1, M2) or a hybrid SARS‐CoV‐2‐S containing the intracytoplasmic domain of VSV‐G (M3, M4). (B) Mice were boosted with the expression plasmids used for priming (M1, M3), soluble trimeric S protein (M2), or exosomes carrying the hybrid SARS‐CoV‐2‐S protein (M4). (C) A flow cytometric assay assessed the binding of sera at a 1:200 dilution to the SARS‐CoV‐2‐S protein with HEK‐293T cells transiently expressing the S protein. Numbers indicate the relative mean fluorescence intensities of sera drawn 2 weeks after the booster immunizations. (D) Competitive inhibition of hACE2‐Fc binding to trimeric S protein by sera (1:200) from control mice and mice at the indicated time points after the first immunization. The mean percentage of binding compared to control binding is shown (two experiments each performed in triplicates). (E) For the neutralization assay, Vero‐E6 cells were infected with the SARS‐CoV‐2 isolate MUC‐IMB‐1 in the presence or absence of week 5, sera, and three control sera from TRIANNI mice immunized with an irrelevant immunogen. SARS‐CoV‐2 infection was quantitated after 20 to 24 h by staining with purified IgG from a convalescent COVID‐19 patient and a fluorescence‐labeled anti‐human IgG using an ELISPOT reader. The mean and SEM of triplicates of one experiment are shown. The control sera represent the mean and SEM of the mean of three control sera each tested in triplicates.
