Abstract
Background
Hospitalized patients with COVID‐19 have increased risks of venous (VTE) and arterial thromboembolism (ATE). Active cancer diagnosis and treatment are well‐known risk factors; however, a risk assessment model (RAM) for VTE in patients with both cancer and COVID‐19 is lacking.
Objectives
To assess the incidence of and risk factors for thrombosis in hospitalized patients with cancer and COVID‐19.
Methods
Among patients with cancer in the COVID‐19 and Cancer Consortium registry (CCC19) cohort study, we assessed the incidence of VTE and ATE within 90 days of COVID‐19–associated hospitalization. A multivariable logistic regression model specifically for VTE was built using a priori determined clinical risk factors. A simplified RAM was derived and internally validated using bootstrap.
Results
From March 17, 2020 to November 30, 2020, 2804 hospitalized patients were analyzed. The incidence of VTE and ATE was 7.6% and 3.9%, respectively. The incidence of VTE, but not ATE, was higher in patients receiving recent anti‐cancer therapy. A simplified RAM for VTE was derived and named CoVID‐TE (Cancer subtype high to very‐high risk by original Khorana score +1, VTE history +2, ICU admission +2, D‐dimer elevation +1, recent systemic anti‐cancer Therapy +1, and non‐Hispanic Ethnicity +1). The RAM stratified patients into two cohorts (low‐risk, 0–2 points, n = 1423 vs. high‐risk, 3+ points, n = 1034) where VTE occurred in 4.1% low‐risk and 11.3% high‐risk patients (c statistic 0.67, 95% confidence interval 0.63–0.71). The RAM performed similarly well in subgroups of patients not on anticoagulant prior to admission and moderately ill patients not requiring direct ICU admission.
Conclusions
Hospitalized patients with cancer and COVID‐19 have elevated thrombotic risks. The CoVID‐TE RAM for VTE prediction may help real‐time data‐driven decisions in this vulnerable population.
Keywords: clinical decision rules, COVID‐19, SARS‐CoV‐2, thrombosis, venous thromboembolism
Essentials
-
•
The exact risk for venous thromboembolism (VTE) in patients with cancer and COVID‐19 is unknown.
-
•
We assessed the VTE incidence and derived a risk assessment model (RAM) in the CCC19 consortium.
-
•
Hospitalized patients with both active cancer and COVID‐19 have elevated risk of VTE (7.6%).
-
•
A newly derived VTE RAM on admission (CoVID‐TE) can risk stratify patients (11.3% vs. 4.1%).
Alt-text: Unlabelled Box
1. INTRODUCTION
Numerous studies have demonstrated a complex interplay between inflammation and coagulation associated with COVID‐19 that results in an increased risk of venous thromboembolism (VTE) and arterial thrombotic events (ATE).1., 2., 3., 4. Specifically, the exact incidence of VTE associated with COVID‐19 is debated and has ranged from as low as 1% in the general wards to as high as 69% in intensive care units (ICUs) in published reports, depending on the diagnostic approach used and whether screening was performed.5., 6. The link between coagulopathy and COVID‐19 has led to an international collaborative effort of randomized controlled studies designed to investigate the use of anticoagulant therapy to prevent complications associated with COVID‐19 among hospitalized medical inpatients, of which the interim unpublished results were recently released.7
Both cancer and anti‐cancer therapies are well‐known risk factors for thrombotic events.8., 9. While many risk factors have been identified for VTE in patients with cancer, advanced disease as well as certain cancer types such as neoplasms of the pancreas, esophagus, and stomach carry the highest risk.10., 11. Moreover, patients with cancer have a higher incidence of VTE compared to acutely ill medical patients without cancer.12., 13. Despite being a well‐known phenotype for thrombosis, cancer diagnosis and anti‐cancer therapy have not yet been identified as a strong risk factor for COVID‐19–associated thrombosis and the exact thrombotic risk in hospitalized patients with both cancer and COVID‐19 remains unknown. In addition, patients with cancer not only have a higher risk of VTE but also have a higher risk of bleeding on anticoagulation compared to patients without cancer.14., 15. A better understanding of the epidemiology and risk factors of thrombosis in patients with cancer and COVID‐19 will also help researchers, clinicians, and policymakers to place results from the beforementioned randomized controlled trials in a relevant context and help discuss appropriate thromboprophylaxis in more vulnerable patients.
The current study has two aims. First, we aim to estimate the 90‐day incidence of VTE and ATE for patients with COVID‐19 and cancer requiring hospitalization, stratified by ICU status and active cancer status. Second, we aim to derive a simple risk assessment model (RAM) specifically for VTE at the time of hospital admission.
2. METHODS
2.1. Study design
The COVID‐19 and Cancer Consortium registry (CCC19; NCT04354701) is an ongoing multi‐center effort to assess the clinical‐pathologic factors and disease course among patients with COVID‐19 and either a current or previous diagnosis of cancer. Details of the original study design and data capture have been reported previously and are available publicly.16., 17., 18. Briefly, data were captured at baseline around the time of COVID‐19 diagnosis and then at 30 days, 90 days, and 180 days after diagnosis. Centralized data management is coordinated through REDCap at Vanderbilt University. Given the de‐identified nature of the data collected, this study has been exempted from institutional review board (IRB) review at Vanderbilt University.
2.2. Cohort selection
Adult patients with an active or previous diagnosis of cancer with a laboratory‐confirmed SARS‐CoV‐2 test from March 17, 2020 to November 30, 2020 were included in the current study. Patients were excluded if they did not reside within United States or Canada, did not have assessable thrombotic complication status within 90 days (13 weeks), were never hospitalized at baseline, had poor data quality (quality score ≥5, typically due to very high levels of missingness),19 or had follow‐up less than 30 days (interval between the COVID‐19 diagnosis and the analysis data lock).
2.3. Outcome definitions
The primary outcomes included VTE as defined by pulmonary embolism (PE), deep vein thrombosis (DVT), or thrombosis not otherwise specified (NOS); and ATE as defined by myocardial infarction (MI) or ischemic stroke (CVA). Secondary outcomes included frequency of PE and/or DVT (excluding thrombosis NOS), PE only, or CVA only. All thrombotic complications were captured as binary “yes/no” responses through retrospective chart review. The exact definition (imaging vs. clinical diagnosis, proximal vs. distal, symptomatic vs. incidental) was left to the discretion of individual sites. Notably, superficial venous thrombosis (SVT) was captured separately and was not included in any of the above definitions.
2.4. Prognostic risk factors
Members of the thrombosis research working group within the CCC19 defined important prognostic risk factors for VTE in hospitalized medical patients with both cancer and COVID‐19 using previously published data from general medical inpatients,20 patients with cancer,10 and patients with COVID‐195 (Table S1 in supporting information). Specifically, the following clinical variables were chosen a priori at the time of admission as potentially important covariates based on plausibility and literature: age at COVID‐19 diagnosis, sex, race/ethnicity, morbid obesity with body mass index (BMI) ≥35, history of VTE, cancer type VTE risk according to the original Khorana Score,10 cancer status (active vs. previous), any recent anti‐cancer systemic therapy within prior 3 months, antiplatelet medication prior to admission, anticoagulant medication prior to admission, or severe COVID‐19 disease requiring direct ICU admission. Additional laboratory values at the time of admission were included in an exploratory analysis: white blood cell count, hemoglobin, platelet count, and D‐dimer. Of note, we chose pre‐admission anticoagulant use instead post‐admission prophylaxis/therapeutic use to enable the calculation of risk factors at the time of admission.
2.5. Statistical methods
The cumulative incidence of VTE and ATE within 90 days after COVID‐19–associated admission was determined by the number of reported VTE or ATE events from data forms within 13 weeks of follow‐up divided by the number of total available patients that met the inclusion and exclusion criteria. The primary and secondary outcomes were further estimated in pre‐specified subgroups (ICU vs. wards, recent systemic therapy vs. none). The incidence trend was also plotted over quarterly intervals for the year 2020. As neither thrombotic nor mortality events had associated time stamps to protect patient identity, we did not perform competing risk analyses.
To derive a prognostic RAM for VTE, we built a multivariable logistic regression model to assess the association between 90‐day VTE outcomes and baseline covariates. We included all pre‐specified clinical covariates in a single model. With the exception of age, all other covariates were categorical. Age was explored both as a continuous linear variable and cubic splines. Additionally, interaction between age and other covariates was checked using the likelihood ratio test. Adjusted odds ratios (OR) and 95% confidence intervals (CI) for VTE and PE were estimated from the models. The relative strengths of each predictor within the model were assessed using the model chi‐square statistic. Multiple imputation through 10 iterations with additive regression, bootstrapping, and predictive mean matching was used to impute missing/unknown data for all clinical variables with <5% missingness in the primary analysis or laboratory variables with <10% missingness in the sensitivity analysis.
To create a simplified RAM, we used the strongest predictors from the multivariable model and assigned simplified integer scores based on the ratio from the division of the covariate’s beta coefficient by the lowest beta coefficient. Only patients with non‐missing values in all predictor categories were included in this analysis. Final risk categories were created using the sum of individual integer scores. The overall goodness‐of‐fit of all models was checked using the Hosmer‐Lemeshow (HL) test and calibration plot. Internally validated discrimination was performed using the optimism‐corrected c‐statistic, for which optimism was calculated as the mean difference in c‐statistic between the original and 1000 bootstrapped resamples.
Several exploratory and sensitivity analyses were performed. First, the model was tested after exclusion of patients who were already receiving anticoagulation prior to admission. Second, the model was assessed in patients not requiring ICU admission at the time of hospitalization. Third, it was expanded to explore the additive values of key laboratory values. Finally, we examined the impact of the final RAM on overall bleeding (defined as major, clinically relevant non‐major, or minor bleeding without other specification) and 30‐day mortality. Data analysis was performed in R 4.0.3 (R Foundation for Statistical Computing).
3. RESULTS
3.1. Cohort selection and patient characteristics
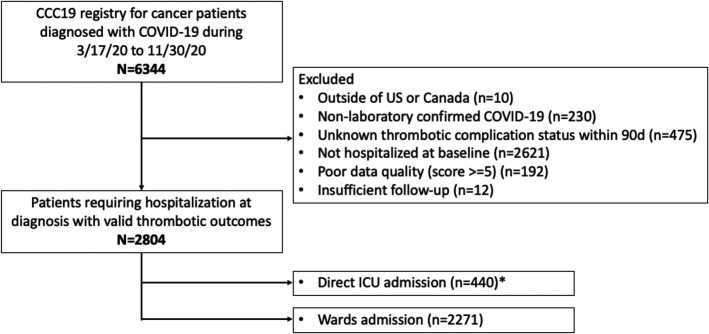
A total of 6344 patients were recorded in the CCC19 database between March 17, 2020 and November 30, 2020. After exclusion, 2804 patients with cancer and COVID‐19 diagnosis who required hospitalization at diagnosis with valid thrombotic outcomes captured were included in the current study (Figure 1 ). Among this hospitalized cohort, 16% (n = 440) were admitted directly to the ICU and 81% (n = 2271) were initially admitted to non‐ICU medical wards (3% had unknown status). The median follow‐up was 42 days (interquartile range [IQR] 21–90). Unless death had occurred prior to follow‐up time point assessment, approximately 83% patients had the 30‐day follow‐up form completed and 62% patients had the 90‐day follow‐up form completed.
FIGURE 1.
Patient selection for study inclusion and exclusion. This flow diagram indicates the inclusion and exclusion criteria for patient selection for the current study using the CCC19 consortium. * Some patients had unknown ICU admission status. CCC19, COVID‐19 and Cancer Consortium registry; ICU, intensive care unit
The median age of patients was 70 (IQR 60–79) and 54% (n = 1504) were male (Table 1 ). Racial/ethnic breakdown revealed 48% (n = 1351) non‐Hispanic White patients, 25% (n = 689) non‐Hispanic Black patients, 13% (n = 368) Hispanic patients, and 12% (n = 345) other. Approximately 74% (n = 2079) had solid tumors, 48% (n = 1342) had disease in remission, and 36% (n = 1021) received systemic anti‐cancer therapy within the 3 months prior to COVID‐19 diagnosis. The distribution of cancer subtypes is shown in Table S2 in supporting information. Among them, 3% (n = 73) had very high‐risk VTE malignancy (pancreatic, esophageal, stomach), 23% (n = 641) had high‐risk VTE malignancy (lung, ovarian, kidney, bladder, testicular, lymphoma), and 75% (n = 2090) had low‐risk VTE malignancy (all others). Approximately 15% (n = 429) of patients were reported to have morbid obesity. D‐dimer was measured in 58% (n = 1623) of patients and a significant majority had abnormal value (n = 1376, 85%). Eleven percent of patients (n = 297) had prior history of VTE, 21% (n = 584) were taking anticoagulants, and 34% (n = 949) were taking antiplatelets prior to COVID‐19 diagnosis. During the COVID‐19 admission, 53% (n = 1473) received anticoagulation for prophylaxis, 13% (n = 367) received anticoagulation for therapeutic reasons, 22% (n = 609) received no anticoagulation, and 13% (n = 355) had unknown status.
TABLE 1.
Demographics and baseline characteristics for hospitalized patients with cancer and COVID‐19
| Hospitalized patients | |
|---|---|
| Total number, N | 2804 |
| Age in years, median (IQR) | 70 (60–79) |
| Male sex, % (N) | 54% (1504) |
| Race/ethnicity, % (N) | |
| White | 48% (1351) |
| Black | 25% (689) |
| Hispanic | 13% (368) |
| Other | 12% (345) |
| Unknown/missing | 2% (51) |
| Cancer subtype, % (N)a | |
| Solid | 74% (2079) |
| Hematologic | 24% (676) |
| Other | 2% (49) |
| Cancer status, % (N) | |
| Remission/no evidence of disease | 48% (1342) |
| Active, stable or responding | 26% (742) |
| Active, progressing or unknown | 23% (651) |
| Unknown/missing | 2% (69) |
| Cancer staging, % (N) | |
| Localized | 50% (1405) |
| Disseminated | 29% (812) |
| Unknown/missing | 21% (587) |
| Recent systemic therapy last 3 months, % (N) | |
| No | 61% (1717) |
| Yes | 36% (1021) |
| Unknown/missing | 2% (66) |
| VTE risk by cancer subtype, % (N)b | |
| Low‐risk VTE malignancy | 75% (2090) |
| High‐risk VTE malignancy | 23% (641) |
| Very high‐risk VTE malignancy | 3% (73) |
| History of VTE, % (N) | |
| No | 89% (2488) |
| Yes | 11% (297) |
| Unknown/missing | 1% (19) |
| Morbid obesity (BMI>35), % (N) | |
| No | 84% (2359) |
| Yes | 15% (429) |
| Unknown/missing | 1% (16) |
| Anticoagulant use prior to admission, % (N) | |
| No | 76% (2136) |
| Yes | 21% (584) |
| Unknown/missing | 3% (84) |
| Antiplatelet use prior to admission, % (N) | |
| No | 63% (1761) |
| Yes | 34% (949) |
| Unknown/missing | 3% (84) |
| Direct ICU admission, % (N) | |
| No | 81% (2271) |
| Yes | 16% (440) |
| Unknown/missing | 3% (93) |
| White blood cell (WBC), % (N) | |
| Within normal limit of normal | 58% (1614) |
| Below lower limit of normal | 20% (552) |
| Above higher limit of normal | 17% (468) |
| Unknown/missing | 10% (173) |
| Hemoglobin (Hb), % (N) | |
| Within normal limit of normal | 37% (1034) |
| Below lower limit of normal | 55% (1552) |
| Above higher limit of normal | 2% (45) |
| Unknown/missing | 6% (173) |
| Platelet (Plt), % (N) | |
| Within normal limit of normal | 61% (1709) |
| Below lower limit of normal | 27% (757) |
| Above higher limit of normal | 4% (125) |
| Unknown/missing | 8% (213) |
| D‐dimer (DD), % (N) | |
| Within normal limit of normal | 9% (247) |
| Above higher limit of normal | 49% (1376) |
| Not tested/not available | 37% (1034) |
| Unknown/missing | 5% (147) |
Abbreviations: BMI, body mass index; ICU, intensive care unit; IQR, interquartile range; VTE, venous thromboembolism.
Please refer to Table S1 for detailed cancer type breakdown.
Adapted from Khorana Score: very‐high risk = pancreas, stomach, esophageal; high risk = lung, ovarian, kidney, bladder, testicular, lymphoma.
3.2. Incidence of VTE and ATE by illness severity and systemic therapy subgroups
Among hospitalized patients, VTE occurred in 7.6% (n = 213) patients, of which 4.0% (n = 113) were PE; ATE occurred in 3.9% (n = 109) patients, of which 1.6% (n = 45) were CVA. Most VTE and ATE events occurred within 30 days of hospitalization. The incidence remained nearly constant from the second to the fourth quarter of 2020 (Figure S1 in supporting information).
In the prespecified subgroup analysis (Table 2 ), the incidence of all thrombotic complications was approximately 2‐fold higher among severely ill patients requiring direct ICU admission (VTE 14.1%, ATE 7.3%) compared to moderately ill patients requiring wards admission (VTE 6.3%, ATE 3.2%). The incidence of VTE but not ATE was higher among patients receiving recent anti‐cancer systemic therapy (VTE 10.0%, ATE 3.1%) compared to those not receiving recent therapy (VTE 5.8%, ATE 4.0%). There was no significant interaction between the two subgroups and the risk factors appeared to be multiplicative.
TABLE 2.
Incidence of venous thrombosis (VTE, PE/DVT, PE) and arterial thrombosis (ATE, CVA) in cancer patients within 90 days post‐SARS‐CoV‐2 diagnosis and hospitalization, stratified by ICU admission & anti‐cancer treatment
| VTE | PE/DVT | PE | ATE | CVA | |
|---|---|---|---|---|---|
| Hospitalized patients with cancer and COVID (n = 2804) | 7.6% (213) | 6.6% (186) | 4.0% (113) | 3.9% (109) | 1.6% (45) |
| ICU admission statusa | |||||
| Direct ICU admission (n = 440) | 14.1% (62) | 12.3% (54) | 7.5% (33) | 7.3% (32) | 2.3% (10) |
| Wards admission (n = 2271) | 6.3% (143) | 5.5% (126) | 3.3% (75) | 3.2% (72) | 1.4% (31) |
| Recent anti‐cancer therapyb | |||||
| Recent systemic therapy (n = 1021) | 10.0% (102) | 8.9% (91) | 5.7% (58) | 3.1% (32) | 1.4% (14) |
| No recent therapy (n = 1717) | 5.8% (99) | 4.8% (83) | 2.6% (45) | 4.0% (69) | 1.7% (29) |
Note
VTE = venous thromboembolism defined as pulmonary embolism (PE), deep vein thrombosis (DVT), or thrombosis not otherwise specified (i.e., unusual splanchnic or cerebral sinus venous thrombosis), ATE = arterial thromboembolism defined as myocardial infarction or ischemic stroke (CVA).
Abbreviation: ICU, intensive care unit.
There are 93 patients with unknown/missing ICU admission status.
There are 66 patients with unknown/missing recent anti‐cancer status.
3.3. Multivariable modeling of VTE and PE risk among hospitalized patients with cancer and COVID‐19
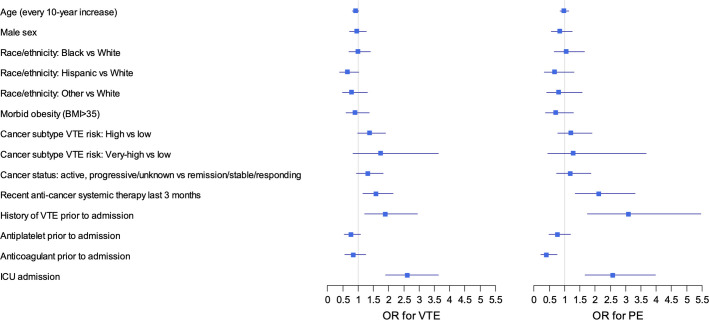
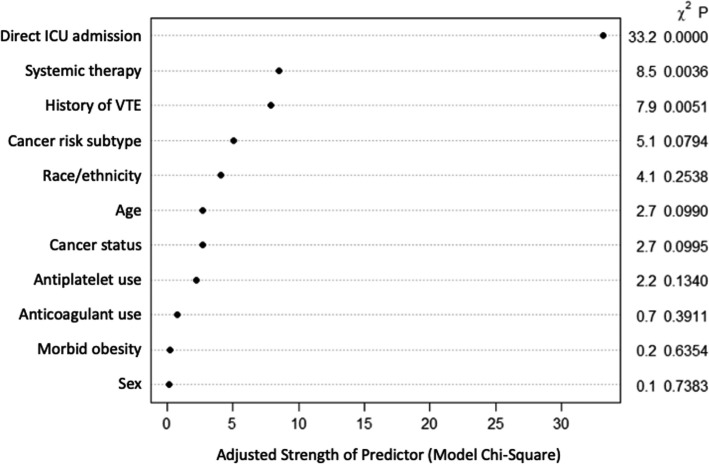
Variables significantly associated with VTE (primary outcome) included recent anti‐cancer systemic therapy (OR 1.58, 95% CI 1.16–2.14), VTE history (OR 1.89, 95% CI 1.21–2.95), and direct ICU admission (OR 2.62, 95% CI 1.89–3.64; Figure 2 ). Other non‐significant but appreciable variables with notable degrees of association based on model chi‐square test (Figure 3 ) included cancer subtype VTE risk (OR 1.37, 95% CI 0.99–1.89 for high risk vs. low risk; OR 1.74, 95% CI 0.83–3.64 for very‐high risk vs. low risk) and Hispanic race/ethnicity (OR 0.64, 95% CI 0.39–1.03 for Hispanic vs. White). In the current study, Black patients represented 25% of the population but did not have an appreciably increased VTE risk compared to White patients (OR 0.99, 95% CI 0.70–1.40). The c statistic was 0.67 (bootstrapped 95% CI 0.63–0.70). The model had adequate fit as demonstrated by an HL test P‐value of .48 and the appearance of the calibration plot (Figure S2 in supporting information).
FIGURE 2.
Forest plot for multivariable logistic regression analysis for association between potential clinical variables and venous thromboembolism (VTE) and pulmonary embolism (PE; n = 2804). This forest plot shows the adjusted odds ratios (OR) for either VTE or PE for each of the chosen clinical covariates. * Adapted from Khorana Score: very‐high risk = pancreas, stomach, esophageal; high risk: lung, ovarian, kidney, bladder, testicular, lymphoma
FIGURE 3.
Relative importance of variables in the predictive model. This figure shows the relative strengths of each predictor within the final multivariable model assessed via the model chi‐square statistics. VTE, venous thromboembolism
The multivariable model for PE (secondary outcome) showed a similar pattern of associations (Table S3 in supporting information). The additional variable that reached significant association was anticoagulant use prior to admission (OR 0.40, 95% CI 0.22–0.75). The PE‐specific model had similar performance as the VTE model with a discrimination c statistic of 0.69 (bootstrapped 95% CI 0.64–0.74) and an HL P‐value of .13. Dedicated association testing for ATE outcomes was not performed due to the low number of events.
3.4. Scenario and sensitivity analyses for VTE predictors
We performed several additional scenario and sensitivity analyses. In the scenarios in which we retested the clinical model after excluding patients on anticoagulants prior to admission (Table S4 in supporting information) or excluding severely ill patients requiring ICU admission (Table S5 in supporting information), the models retained very similar magnitude and significance for its list of associated factors. In a separate sensitivity analysis after adding laboratory values to the clinical model, elevated D‐dimer and platelet count were significantly associated with VTE, but the overall c‐statistic was not appreciably different (Table S6 in supporting information).
3.5. CoVID‐TE simplified risk assessment score
Based on the key predictors from the multivariable model, we created a simplified prediction score by assigning integer weights proportional to the beta coefficients. From the original cohort of 2804 patients, 2457 had complete data capture for all the chosen baseline variables (no imputation). Variables with their associated weights included: Cancer subtype high to very‐high risk by original Khorana score (pancreas, stomach, esophageal, lung, ovarian, kidney, bladder, testicular, lymphoma; +1), VTE history (+2), ICU admission (+2), D‐dimer elevated on admission (+1), Therapy (recent systemic therapy last 3 months) (+1), and Ethnicity non‐Hispanic (+1; Table 3 ). The initial letter of each variable formed the new risk assessment model “CoVID‐TE” for COVID‐19–associated thromboembolism. Patients with scores 0 to 2 (n = 1423) appeared to have lower risk of VTE (4.1%) and PE (2.3%). In contrast, patients with a score of 3 or higher (n = 1034) had appreciably increased risk of VTE (11.3%) and PE (5.5%). The simplified RAM had modest discrimination with a c statistic of 0.67 (0.63–0.71) for VTE prediction and an HL test P‐value of .90. In a sensitivity analysis, after excluding patients with anticoagulant use prior to COVID‐19 diagnosis, the CoVID‐TE RAM demonstrated similar discrimination, with a c‐statistic of 0.69 (0.64–0.74) for VTE prediction (Table S7 in supporting information). Finally, we assessed overall bleeding and 30‐day mortality for patients stratified by the CoVID‐TE RAM (Table S8 in supporting information). Compared to patients with low risk for VTE, those classified as high risk for VTE also had higher risk for overall bleeding (10.0% vs. 4.3%) and mortality (29.3% vs. 19.5%). As the bleeding endpoint was not predefined or adjudicated, this remained an exploratory analysis.
TABLE 3.
Simplified risk assessment model for VTE (CoVID‐TE thromboembolism score) in hospitalized patients with complete data. (a) CoVID‐TE score assignment, (b) CoVID‐TE risk category stratification and performance
| Risk assesment model | |
|---|---|
| (a) | |
| Baseline variables | Point |
| Cancer subtype by original Khorana scorea | +1 |
| VTE history (lifetime) | +2 |
| ICU triage on admission | +2 |
| D‐dimer elevatedb | +1 |
| Therapy (recent systemic last 3 months) | +1 |
| Ethnicity non‐Hispanicc | +1 |
| (b) | ||
|---|---|---|
| All hospitalized patients (n = 2457) | ||
| Risk category (N) | VTE % (N) | PE % (N) |
| Low‐risk (0–2) | 4.1% (59) | 2.3% (33) |
| 0–1 (657) | 3.6% (24) | 2.0% (13) |
| 2 (766) | 4.6% (35) | 2.6% (20) |
| High‐risk (3+) | 11.3% (117) | 5.5% (57) |
| 3 (529) | 8.9% (47) | 3.6% (19) |
| 4 (317) | 11.7% (37) | 6.3% (20) |
| 5+ (188) | 17.6% (33) | 9.6% (18) |
| C statistic (95% CI) | 0.67 (0.63–0.71) | 0.67 (0.61–0.73) |
| HL test p‐value | .90 | .77 |
Note
Integer points (1 or 2 points) were assigned to each of the baseline variables listed in the table above. A final composite score ranging from 0 to 8 is created. Based on outcome distribution shown above, 0–2 point is considered low‐risk and 3 or more points is considered high‐risk.
Abbreviations: HL, Hosmer‐Lemeshow test; ICU, intensive care unit; PE, pulmonary embolism; VTE, venous thromboembolism.
Combined high and very‐high risk categories based on the original Khorana score: pancreas, stomach, esophageal, lung, ovarian, kidney, bladder, testicular, lymphoma.
Specific cut‐off could not be determined using the current dataset.
Asian race also associated with lower risk of VTE in other studies; however, this was not specifically assessed in the current study.
4. DISCUSSION
In this retrospective cohort study of 2804 hospitalized patients with COVID‐19 and cancer, we found the 90‐day VTE and ATE incidences were elevated at 7.6% (n = 213) and 3.9% (n = 109), respectively. VTE risk was higher in patients admitted to ICU and those with active cancer having received recent systemic therapy. Our newly derived VTE risk assessment model, the CoVID‐TE score, incorporated six clinicopathologic factors readily available at the time of hospital admission. With a modest discrimination, the CoVID‐TE score stratified patients into two different risk categories: 58% in the low‐risk group (score 0–2) had an observed incidence of 4.1% for VTE and 2.3% for PE; 42% in the high‐risk group (score 3+) had an incidence of 11.3% for VTE and 5.5% for PE. Patients with these thrombotic risk factors might also be at higher risk for bleeding and mortality. We believe risk stratification from the current study can provide relevant context and help with the interpretation and implementation of anticoagulant prophylaxis based on the results of ongoing randomized controlled trials for this vulnerable patient population.
While COVID‐19, prolonged hospitalization, and active cancer are all well‐known pro‐thrombotic risk factors, the exact incidence of thrombosis in a population with all three has not been previously reported. We examined the incidence over a 90‐day follow‐up to match with previous studies for medical inpatient‐associated thrombosis.20 Among general patients hospitalized with COVID‐19, the reported incidence based on retrospective cohort studies varies significantly depending on the geographic region, acute care setting, anticoagulation preference, and study outcome definitions.21 Within the United States, the reported incidence of VTE ranges from 1% to 4% and ATE (excluding biomarker‐only definition of MI similar to our study) ranges from 1% to 2% for patients admitted to the wards.22., 23., 24., 25. In contrast, those admitted to the ICU have a reported incidence of VTE ranging from 8% to 14% and ATE ranging from 6% to 8%.22., 23., 24., 25., 26. In the current study, we observed a similar incidence of hospital‐associated thrombosis (VTE 4.5% in wards, 12.2% in ICU; ATE 3.2% in wards, 8.1% in ICU) in patients with history of cancer but not on active therapy (many of whom were in remission or had no current evidence of cancer). This finding suggests that a historical diagnosis of cancer alone may not be a significant risk factor for thrombosis. In contrast, patients with cancer and COVID‐19 recently receiving systemic therapy had significantly higher than expected incidence for VTE (9.0% in wards, 15.8% in ICU) but not ATE (2.7% in wards, 4.8% in ICU). The additive effects from active infection and active anti‐cancer treatment may cause these patients to be at particularly high risk for hospital‐associated VTE. Of note, we excluded patients not requiring hospitalization at the time of COVID‐19 diagnosis as their information might not be fully captured and their outcomes might be under‐reported. Nonetheless, we observed very low thrombotic rates with 0.7% VTE and 0.2% ATE at the same 90‐day follow‐up window among outpatients in the CCC19 registry (excluded from this study).
In addition to reporting the incidence of thrombosis, the highlight of the current work is the derivation of a parsimonious RAM using the six important clinical variables. To facilitate ease of use, we focused on simplicity and availability at presentation to the hospital. Consistent with previously published data outside of the COVID‐19 literature, we found that ICU admission, recent systemic therapy, history of VTE, cancer VTE risk subtype, non‐Hispanic race/ethnicity, and active cancer status were the strongest clinical risk factors associated with VTE. Among the laboratory‐based variables, both thrombocytosis and elevated D‐dimer were associated with increased VTE. We chose to use D‐dimer, as it is now more commonly measured in the hospitalized patient with COVID‐1927 and when assessed prior to the pandemic, it was specifically superior to thrombocytosis as a biomarker for VTE risk prediction.28
Our findings are especially pertinent given the interim results of a combined multiplatform international randomized control trial that assessed the role of therapeutic anticoagulation among hospitalized patients with COVID‐19 on the need for organ support and secondarily on mortality.7 Given the significant variability that is present in the baseline clinical characteristics and the relative rare prevalence of cancer in the general population, there is likely going to be considerable heterogeneity of treatment effect (THE) with therapeutic anticoagulation. Our RAM focuses exclusively on patients with cancer and demonstrated reasonable discrimination for VTE with good calibration. In keeping with the recently developed Predictive Approaches to Treatment effects Heterogeneity (PATH) consensus for exploring THE across trial populations,29 we believe this novel risk prediction tool could provide the initial rationale for a risk modeling approach to exploring the THE that is inevitable in a trial of patients with COVID‐19.
There are several strengths and limitations to our study. Our study was one of the largest series from more than 128 institutions to examine the thrombotic complications among hospitalized patients with cancer and COVID‐19. Due to the sample size, we were able to apply stringent selection criteria to ensure adequate follow‐up and exclude patients with incomplete data capture such that multiple imputation was only performed to address missing data in variables with <5% missingness (or <10% in exploratory analyses). Our multiple sensitivity analyses showed that the findings would be replicable under different meaningful clinical scenarios. Furthermore, the CoVID‐TE RAM consists of variables that are simple and readily available to providers at the time of admission with the potential to impact the care of patients with cancer and COVID‐19 in a meaningful way. Limitations inherent to our non‐randomized retrospective study nature include the potential for unmeasured confounding, selection bias, and underreporting of outcomes. Given that each institution had its own protocol for the prevention and diagnosis of thrombosis, this heterogeneity could have impacted the actual rates from individual sites, although the stable aggregate rates of VTE over each quarter of 2020 verified overall consistency. Furthermore, the heterogeneous use of anticoagulation before admission (21%) and the low proportion of very high‐risk cancer types (3%) may limit the generalizability of the RAM. Additionally, we did not perform time‐to‐event or competing risks analysis due to the lack of specific timing for VTE complications. Finally, for any novel model to be clinically applicable, it needs to be tested and validated in an external cohort.
In conclusion, we investigated the incidence of venous and arterial thromboses in hospitalized patients with cancer and COVID‐19 and derived a new RAM that can be calculated at the time of admission to risk stratify patients into different VTE risk groups. We anticipate that the CoVID‐TE RAM, upon external validation, can serve as a real‐time clinical decision support tool to assist with personalized decisions on the initiation of thromboprophylaxis in hospitalized patients with cancer and COVID‐19.
CONFLICTS OF INTEREST
ARK (or an immediate family member) have currently or during the past 2 years owned stock or held an ownership interest in Merck, Sanofi, and BMS. ALS reports travel support from Pfizer and Astellas, outside the scope of the submitted work. BIR reports grants, personal fees, and non‐financial support from Merck; grants and personal fees from BMS; grants and personal fees from Pfizer, grants and personal fees from Aveo, grants from Astra Zeneca, grants and personal fees from Genentech; personal fees from Synthorx; personal fees from 3D Medicines; personal fees from Arravive; personal fees from Surface Oncology; other from PTC Therapeutics; personal fees from Arrowhead Therapeutics, outside the scope of the submitted work. BH reports research funding to the institution from Amgen, Abbvie, BI, Mirati, Merck, Eli‐Lilly, Astra‐Zeneca, BMS, Novartis, GSK, Pfizer, Advaxis, and GuardantHealth; consulting/advisory role with Merck, BMS, Genentech, Astra‐Zeneca, Amgen, Novartis, TPT, VI, GuardantHealth; and honoraria from PER and OncLive, all outside the scope of the submitted work. GLL reports research grant from Amgen (to institution); consulting for BeyondSpring, G1 Therapeutics, Samsung, TEVA, all outside the scope of the submitted work. GdLL reports honoraria from Boehringer Ingelheim; consulting or advisory role for Pfizer and AstraZeneca; research funding from AstraZeneca; funding to his institution from Merck Sharp & Dohme, EMD Serono, AstraZeneca, Blueprint Medicines, Tesaro, Bavarian Nordic, NOVARTIS, G1 Therapeutics, adaptimmune, BMS, GSK, Abbvie, Rgenix, Pfizer, Roche, Genentech, Lilly, Janssen; travel, accommodations, and expenses from Boehringer Ingelheim, Pfizer, E.R. Squibb Sons, LLC, Janssen, all outside the scope of the submitted work. NMK reports personal fees from G1 Therapeutics, Invitae, Beyond Spring, Spectrum, BMS, Janssen, Total Health, all outside the scope of the submitted work. JW reports research grant support from the National Cancer Institute in support of this work; the funder had no role in the design of conduct of the study, or the decision to publish. Equity in HemOnc.org LLC, Consultant: Westat, IBM Watson Health; outside the scope of the submitted work. VK reports honoraria from Diagnostica Stago, all outside the scope of the submitted work. SG reports research grant support to her institution from Astrazeneca and Isoray, all outside the scope of submitted work. RPR reports research grant support to her institution from BMS and Janssen; Consultant/Advisory Board: BMS, Janssen, and Dova, all outside scope of submitted work. JMP reports honoraria from Radius, outside the scope of the submitted work. BH reports research grant support for his work from the NCI (NCORP 2UG1CA189859‐06), research grant support to his institution from Merck, Boehringer‐Ingelheim, Astra‐Zeneca, BMS, Mirati, AbbVie, Advaxis, Pfizer, Novartis, BeiGene, Elevation Oncology, Blueprint and received fees for consulting from Merck, BMS, Novartis, Janssen, Genentech, Astra‐Zeneca, Boehringer‐Ingelheim, Pfizer, TPT, Apollomics, all outside the scope of the submitted work. CAP (or an immediate family member) have currently or during the past 2 years owned stock or held an ownership interest in Pfizer, Epizyme, Inovio, OPKO Health Inc, Roche., all outside the scope of the submitted work. CH reports funding from the Henry Ford Cancer Institute supporting the current work; research funding paid to her institution from Merck, Exelixis, Bayer, AstraZeneca, Genentech, Dendreon, and Bausch; personal fees from Sanofi/Genzyme, Dendreon, Exelixis, Bristol Myers Squibb, Astellas, Medivation, Bayer, and Janssen Scientific; and stock ownership by an immediate family member in Johnson and Johnson, outside of the scope of the submitted work. RRM received research funding from Bayer, Pfizer, Tempus; serves on Advisory Board for AstraZeneca, Bayer, Bristol Myers Squibb, Calithera, Exelixis, Janssen, Merck, Novartis, Pfizer, Sanofi, Tempus; is a consultant for Dendreon, Vividion; serves on the molecular tumor board at Caris, all unrelated to the current scope of work. SG reports research funding to the institution from Astra‐Zeneca and consulting/advisory role with Puma Biotechnology. MAT reports travel support from Syapse; royalties from UpToDate, Connect MDS/AML Registry in Celgene (now owned by BMS), Myeloma Registry in Takeda; stock ownership in Doximity; personal fees from VIA Oncology (now owned by Elsevier ClinicalPath), Adaptive Advisory Board, and GSK. Dr. Thompson is the Local PI for Clinical Trials in AbbVie, BMS, CRAB CTC, Denovo, Research Network, Eli Lilly, LynxBio, Strata Oncology, TG Therapeutics, all outside the submitted work. ZB reports research support from Bristol‐Myers Squibb and Genentech/imCORE; honoraria from UpToDate, all outside the scope of the submitted work. NAP reports honoraria for consulting/advisory boards: Merck, AstraZeneca, BMS, Eli Lilly, Genentech, Pfizer, Amgen, G1 Therapeutics, Xencor, Viosera, Inivata, all outside of the scope of submitted work. SP reports personal fees from Abbvie, Amgen, AstraZeneca, Bayer, Biocartis, Boehringer‐Ingelheim, Bistrol‐Myers Squibb, Clovis, Daiichi Sankyo, Debiopharm, Eli Lilly, F. Hoffmann‐La Roche, Foundation Medicine, Illumina, Janssen, MerckSharp and Dohme, Merck Serono, Merrimack, Novartis, Pharma Mar, Pfizer, Regeneron, Sanofi, Seattle Genetics and Takeda, AstraZeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Eli Lilly, F. Hoffmann‐La Roche, Merck Sharp and Dohme, Novartis, Pfizer, Takeda, Bioinvent; non‐financial support from Sponsored by Amgen, AstraZeneca, Boehringer‐Ingelheim, Bristol‐Meyers Squibb, Clovis, F. Hoffmann‐La Roche, Illumina, Merck Sharp and Dohme, Merck Serono, Novartis, Pfizer, Sanofi, (all fees to institution), outside the submitted work; PG (all unrelated to this study, in the last 3 years) has provided consulting or advisory services for AstraZeneca, Bayer, Bristol Myers Squibb, Clovis Oncology, Dyania Health, Driver, EMD Serono, Exelixis, Foundation Medicine, Genentech/Roche, Genzyme, GlaxoSmithKline, Heron Therapeutics, Immunomedics, Infinity Pharmaceuticals, Janssen, Merck & Co., Mirati Therapeutics, Pfizer, QED Therapeutics, Seattle Genetics, and 4D Pharma PLC; and has received research funding from Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, Debiopharm, GlaxoSmithKline, Immunomedics, Kure It Cancer Research, Merck & Co., Mirati Therapeutics, Pfizer, and QED Therapeutics. YS reports honoraria from Boehringer Ingelheim, AstraZeneca, Novartis, and Eisai; consulting or advisory role for Pfizer, AstraZeneca, Novartis, Roche, Genentech, and Janssen, all outside the scope of the submitted work. AD, AL, AS, C‐YH, DGB, DPS, DKC, DRR, DGS, IJA, JG, JF, ML, SM have nothing to disclose.
AUTHOR CONTRIBUTIONS
AL, NMK, GHL conceived the study. AL, NMK, YS, GHL, RPR designed the methods. AL, CYH, JLW performed the statistical analysis. AL, RPR drafted the manuscript. AL, NMK, CYH, YS, JLM, DS, VK, SS, ARK, JF, SG, RZ, ML, AD, PE, ZB, DKC, CH, IA, RM, JG, AS, BH, MAT, JMP, NAP, DRR, SP, AE, GdLL, DS, PG, ARK, BR, CAP, SM, JMC, GHL, RPR critically reviewed and edited the manuscript. SG, RZ, PE, ZB, DKC, CH, IA, RM, JG, ARK, PG, GHL collected the data and contributed >10% of study outcomes.
Acknowledgments
Hemostasis and Thrombosis Research Society Mentored Research Award
National Hemophilia Foundation Shire Clinical Fellowship Award
Cancer Prevention and Research Institute of Texas RR190104
Footnotes
Manuscript Handled by: Marc Carrier
Final decision: Marc Carrier, 12 July 2021
Funding informationThis work was supported by Texas Cancer Prevention and Research Institute of Texas (RR190104), Hemostasis and Thrombosis Research Society (Mentored Research Award) supported by an independent educational grant from Shire, and National Hemophilia Foundation Shire Clinical Fellowship Award (AL). National Cancer Institute grant P30 CA068485 (SM, BIR, JLW, CYH, YS).
This is an open access article under the terms of the Creative Commons Attribution‐NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Supporting Information
App S1
REFERENCES
- 1.Price L.C., McCabe C., Garfield B., Wort S.J. Thrombosis and COVID‐19 pneumonia: the clot thickens! Eur Respir J. 2020;56:2001608. doi: 10.1183/13993003.01608-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Sullivan J.M., Gonagle D.M.C., Ward S.E., Preston R.J.S., O'Donnell J.S. Endothelial cells orchestrate COVID‐19 coagulopathy. Lancet Haematol. 2020;7:e553–e555. doi: 10.1016/S2352-3026(20)30215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nopp S., Moik F., Jilma B., Pabinger I., Ay C. Risk of venous thromboembolism in patients with COVID‐19: a systematic review and meta‐analysis. Res Pract Thromb Haemost. 2020;4:1178–1191. doi: 10.1002/rth2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiménez D., García‐Sanchez A., Rali P., et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta‐analysis. Chest. 2020;159(3):1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.https://www.medrxiv.org/content/10.1101/2021.05.13.21256846v1. 10.1101/2021.05.13.21256846 [DOI]
- 8.Khorana A.A., Francis C.W., Culakova E., Kuderer N.M., Lyman G.H. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–2346. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 9.Cohen A.T., Katholing A., Rietbrock S., Bamber L., Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population‐based cohort study. Thromb Haemost. 2017;117:57–65. doi: 10.1160/TH15-08-0686. [DOI] [PubMed] [Google Scholar]
- 10.Khorana A.A., Kuderer N.M., Culakova E., Lyman G.H., Francis C.W. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khorana A.A., Kuderer N.M., McCrae K., et al. Cancer associated thrombosis and mortality in patients with cancer stratified by khorana score risk levels. Cancer Med. 2020;9:8062–8073. doi: 10.1002/cam4.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blom J.W., Doggen C.J.M., Osanto S., Rosendaal F.R. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 13.Blom J.W., Vanderschoot J.P.M., Oostindiër M.J., Osanto S., van der Meer F.J.M., Rosendaal F.R. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4:529–535. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 14.Prandoni P., Lensing A.W.A., Piccioli A., et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 15.Hutten B.A., Prins M.H., Gent M., Ginsberg J., Tijssen J.G., Büller H.R. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: a retrospective analysis. J Clin Oncol. 2000;18:3078–3083. doi: 10.1200/JCO.2000.18.17.3078. [DOI] [PubMed] [Google Scholar]
- 16.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID‐19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera D.R., Peters S., Panagiotou O.A., et al. Utilization of COVID‐19 treatments and clinical outcomes among patients with cancer: a COVID‐19 and cancer consortium (CCC19) cohort study. Cancer Discov. 2020;10:1514–1527. doi: 10.1158/2159-8290.CD-20-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grivas P., Khaki A.R., Wise‐Draper T.M., et al. Association of clinical factors and recent anti‐cancer therapy with COVID‐19 severity among patients with cancer: a report from the COVID‐19 and cancer consortium. Ann Oncol. 2021;32(6):787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abidi M., Aboulafia D.M., Accordino M.K., et al. COVID‐19 and cancer consortium. a systematic framework to rapidly obtain data on patients with cancer and COVID‐19: CCC19 governance, protocol, and quality assurance. Cancer Cell. 2020;38:761–766. doi: 10.1016/j.ccell.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darzi A.J., Karam S.G., Charide R., et al. Prognostic factors for VTE and bleeding in hospitalized medical patients: a systematic review and meta‐analysis. Blood. 2020;135:1788–1810. doi: 10.1182/blood.2019003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosovsky R.P., Sanfilippo K.M., Wang T.F., et al. Anticoagulation practice patterns in COVID‐19: a global survey. Res Pract Thromb Haemost. 2020;4:969–983. doi: 10.1002/rth2.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID‐19 in a New York City health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al‐Samkari H., Karp Leaf R.S., Dzik W.H., et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moll M., Zon R.L., Sylvester K.W., et al. VTE in ICU patients With COVID‐19. Chest. 2020;158:2130–2135. doi: 10.1016/j.chest.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al‐Samkari H., Gupta S., Leaf R.K., et al. Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically Ill patients With COVID‐19. Ann Intern Med. 2021;174(5):622–‐632. doi: 10.7326/M20-6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spyropoulos A.C., Levy J.H., Ageno W., et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pabinger I., van Es N., Heinze G., et al. A clinical prediction model for cancer‐associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol. 2018;5:e289–e298. doi: 10.1016/S2352-3026(18)30063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kent D.M., van Klaveren D., Paulus J.K., et al. The predictive approaches to treatment effect heterogeneity (PATH) statement: explanation and elaboration. Ann Intern Med. 2020;172:W1–W25. doi: 10.7326/M18-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1