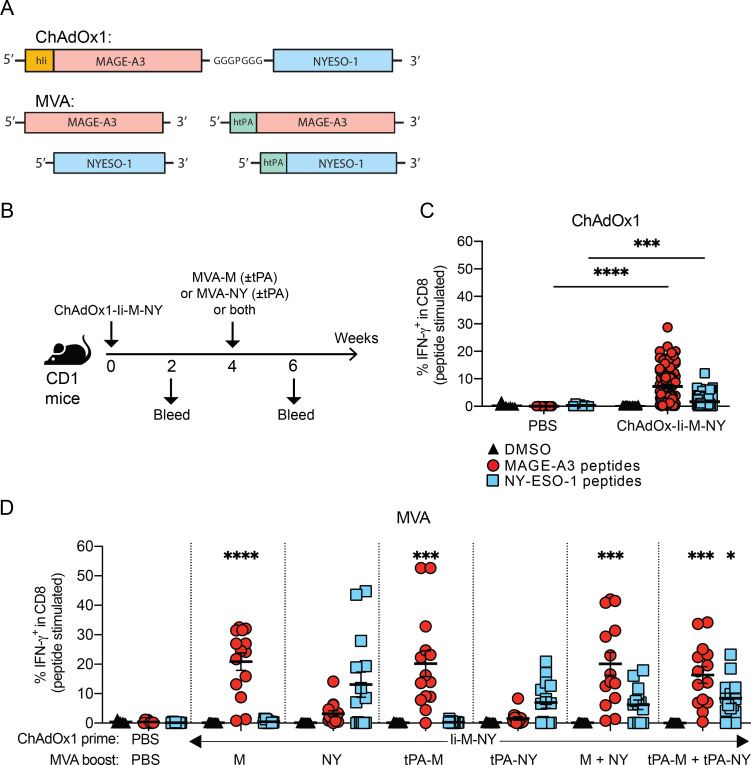
Figure 6.
Evaluation of ChAdOx1/MVA prime-boost vaccination targeting MAGE-A3 and NY-ESO-1. (A) Design of ChAdOx1 and MVA vectors encoding the human MAGE-type antigens MAGE-A3 and NY-ESO-1. (B) CD1 outbred mice were vaccinated via i.m. injection according to the schedule shown. Mice received a prime vaccination with 108 IU of ChAdOx1-Ii-M-NY. Four weeks after ChAdOx1-M-NY, mice received a boost vaccination via a single injection with 107 PFU of either MVA-M (± tPA) or MVA-NY (± tPA), or two injections – one with 107 PFU of MVA-M (± tPA) and the other 107 PFU of MVA-NY (± tPA). To test the response to vaccination, mice were bled 16 days after ChAdOx1 and 14 days after MVA vaccinations. (C-D) Percentage of IFN-γ+ CD8+ T cells in the blood after (C) prime vaccination, (D) and the MVA boost vaccinations. PBMCs were stimulated ex vivo with 4 µg/ml of MAGE-A3 or NY-ESO-1 peptide pools, or a DMSO vehicle control. The percentage of antigen-specific IFN-γ+ -producing CD8+ T cells in the blood after each vaccination was determined by ICS and flow cytometry in response to stimulation with DMSO (black triangles), MAGE-A3 peptides (red circles) or NY-ESO-1 peptides (blue squares). Data are shown as the mean ± SEM and each symbol represents an individual mouse, with 8 mice in the PBS group and 14 mice per ChAdOx1/MVA vaccinated group, pooled from 2 independent experiments. Statistically significant difference is shown compared to the PBS control group and was determined by a Kruskal-Wallis test with Dunn’s multiple comparisons test. *, p ≤ 0.05, **, p ≤ 0.01 ***, p ≤ 0.001, ****, p ≤ 0.0001.