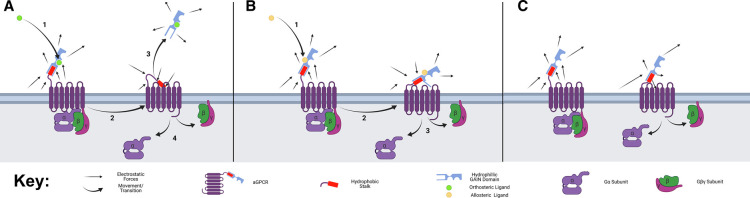
Figure 3. Proposed activation states of aGPCRs and the corresponding electrostatic forces.
Inactive aGPCRs have their G proteins bound and stalks away from the activation domain in the centre of the GPCR. This is due to the hydrophilic GAIN domain still being attached and the hydrophobic stalk being hidden within it. (A) Full activation of the aGPCR is achieved by autoproteolysis of the GAIN domain, to expose the hydrophobic stalk to the ECM, pushing it toward the hydrophobic centre of the activation domain. This activates the GPCR releasing the G protein causing further downstream effects. (B) Partial allosteric activation can result in a conformational change of the GAIN domain resulting in the exposure of part of the hydrophobic stalk. This pushes the stalk toward the activation domain resulting in a higher chance of the G protein subunit dissociating. (C) Some receptors have constitutive activity, and this is likely due to the exposure of some of the hydrophobic residues on the stalk, resulting in more forces pushing the stalk away from the water rich ECM and toward the hydrophobic centre of the aGPCR. This can partially activate the aGPCR resulting in a higher chance of G protein subunit dissociation and downstream effects. Created using Biorender.