Abstract
The solubilisation of membrane proteins (MPs) necessitates the overlap of two contradictory events; the extraction of MPs from their native lipid membranes and their subsequent stabilisation in aqueous environments. Whilst the current myriad of membrane mimetic systems provide a range of modus operandi, there are no golden rules for selecting the optimal pipeline for solubilisation of a specific MP hence a miscellaneous approach must be employed balancing both solubilisation efficiency and protein stability. In recent years, numerous diverse lipid membrane mimetic systems have been developed, expanding the pool of available solubilisation strategies. This review provides an overview of recent developments in the membrane mimetic field, with particular emphasis placed upon detergents, polymer-based nanodiscs and amphipols, highlighting the latest reagents to enter the toolbox of MP research.
Keywords: membrane protein solubilisation, membrane proteins, membrane solubilisation, smalp
Introduction
Membrane proteins (MPs) are indispensable components of biological membranes contributing enormously to both their diverse functionality and structure. Intrinsic membrane proteins (IMPs) are integral to the membrane itself, displaying at least one complete traversion of the phospholipid bilayer. Whilst the assumption of the native 3D structure is inherently determined by the amino acid sequence, a synergistic relationship must be established between membrane-spanning residues and lipids to maintain a stable conformation of the correct oligomeric state. However, structural and functional investigations often require extraction of MPs from the heterogeneous membrane and subsequent reconstitution into an environment which permits such downstream analyses.
The act of MP solubilisation is somewhat paradoxical in that it must simultaneously disrupt the native phospholipid environment whilst also stabilising proteins, possessing extensive hydrophobic regions, as they become liberated into an aqueous habitat. The superposition of these two events represents a major challenge in MP structural and functional studies.
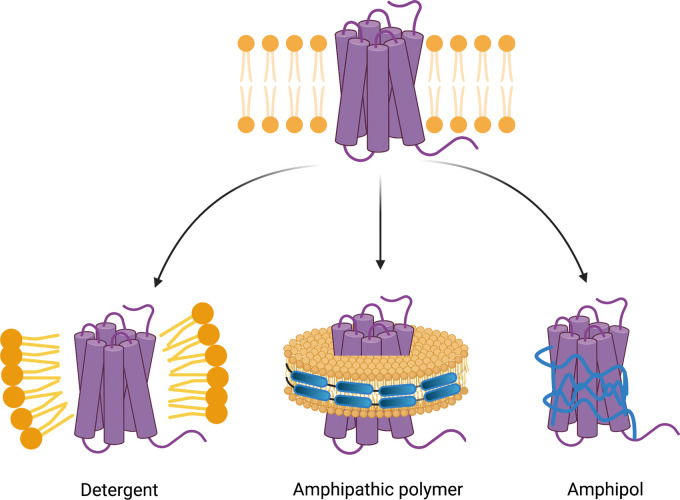
This review aims to provide an overview of the recent innovations in membrane mimetic system innovations, particularly accentuating detergents, polymer-based nanodiscs and amphipols (Figure 1).
Figure 1. Schematic displaying the extraction of MPs into the three membrane mimetic systems discussed in detail in this review.
Left — detergent solubilisation forms a micelle around the hydrophobic region of the MP (purple), middle — solubilisation with a nanodisc forming amphipathic polymer (blue) retains the native membrane lipids (yellow) around the MP, right — amphipols (APols) (blue) self-assemble to mask hydrophobic MP regions from the aqueous environment. Figure created with BioRender.com.
Detergents
The use of detergents, above their critical micelle concentration (CMC), remains the conventional method employed to solubilise MPs prior to biochemical investigation. While the modus operandi of the myriad of available detergents is highly related, there are no golden rules for selecting suitable detergents for the solubilisation of a specific MP. Hence, an exhaustive approach must be employed to identify the optimal detergent, for each individual protein, balancing both solubilisation efficacy and protein stability, however, this is rarely achieved owed to the associated cost and time demands, therefore, a traditional detergent satisfying most of the required needs is often implemented instead.
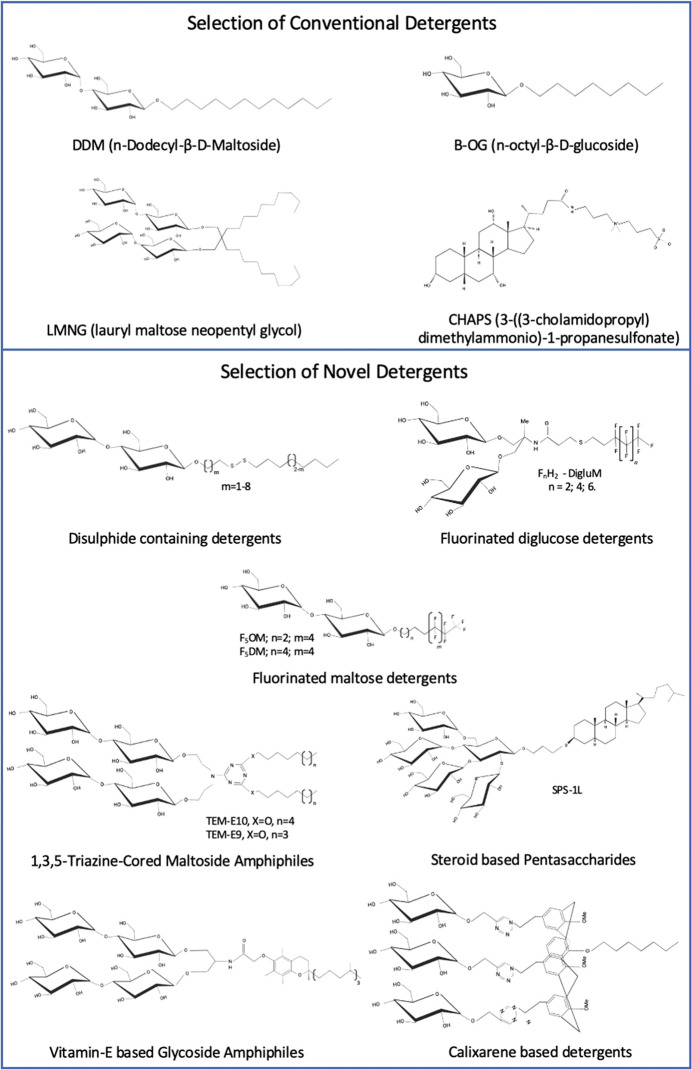
Conventional detergents, such as n-dodecyl-β-D-maltoside (DDM), are typically comprised of a single alkyl chain and hydrophilic group, with few deviating from this architecture (Figure 2). The limited structural miscellany of conventional detergents is in stark contrast with the far-reaching diversity of MPs hence a ‘one size fits all’ approach to detergent selection appears largely impractical. Indeed, certain MPs undergo degradation or loss of function upon incorporation into micelles of these traditional detergents [1–5], thus the development of novel detergents, which may be employed to study a wider array of MPs, continues to attract significant research effort. Past research has focused primarily upon the evolution of detergent classes, via the introduction and modification of functional groups, to confer improved solubilisation/stabilisation properties. However, recently there has been a drive towards developing completely novel detergents that confer, in particular, elevated protein stability, compared with classical detergents. Examples of these include; a new class of steroid-based pentasaccharides [6], fluorinated glucose and maltose-based detergents [7,8], innovative facial amphiphiles (FAs) [9,10], 1,3,5-triazine-cored detergents [11], disulfide containing amphiphiles [12] and modified neopentyl glycol based detergents that have undergone subsequent modification to further improve their characteristics [13] (Figure 2).
Figure 2. Structures of a selection of detergents referred to within this manuscript.
Upper and lower panels depict conventional and novel detergents respectively highlighting the variability within each class and allowing comparison between their architectures.
Unique chemical architectures, deviating significantly from those previously developed, have also been proposed. Indeed, five vitamin-E based glycoside amphiphiles (VEGs), consisting of a hydrophobic vitamin E based alpha-tocopherol chain and hydrophilic branched glycoside head group, were recently developed and characterised to assess their potential biochemical application (Figure 2) [6]. Many MPs, including the β2AR and its Gs complex, displayed elevated stability and retention of functionality over longer incubation periods once incorporated into VEG micelles [6]. Furthermore, negative stain EM of VEG-3 reconstituted β2AR–Gs complex yielded monodisperse, non-aggregated single particles highlighting a potential application in Cryo-EM [6]. Despite these favourable properties, VEGs presented a solubilisation efficiency of approximately 50–60%, much less compared with the gold standard detergent DDM (90%) [6], therefore, even with this latest generation of detergents, the necessity to extract MPs via conventional detergents remains.
Finally, the calixarene platform, formed by the cyclic oligomerisation of four phenol molecules, has provided a promising backbone for the development of novel detergent architectures. Initial calixarene detergents aimed to increase the stability of extracted MPs via interactions between the calixarene platform and both aromatic residues (π-stacking) and basic residues (salt bridges) often found at the cytosolic-membrane interface [14–18]. Subsequent modifications of the hydrophobic and hydrophilic groups appending the central calixarene platform has yielded an ever-increasing array of calixarene detergents. Of note are modifications to the length of the acyl tail which were shown to dramatically influence solubilisation efficacy. Calixarenes possessing short aliphatic tails, less than 7 carbon atoms in length, displayed poor solubilisation abilities, indeed, compounds with 1 and 3 carbon atom tails failed to extract MPs at all [17]. A distinct improvement in solubilisation efficacy was observed for compounds with a tail ≥7 carbon atoms in length with a 12 carbon tailed derivative extracting BmrA and ABCG2 from insect cells with a greater efficiency than DDM [17]. The correlation between longer alkyl chain detergents and improved extraction of MPs has been shown in several previous studies [19–21]. Detergents with longer chains are inherently more hydrophobic, and thus less soluble, than their short-chain counterparts. Consequently, long-chain detergents possess lower CMC values that enable more effective partitioning of MPs into their larger micelles which feature a greater accessible micellar core volume [21]. After probing the solubilisation characteristics of calixarenes, Matar-Merhab et al. [17], concluded that, despite being anionic in nature, these compounds behave in a similar manner to mild detergents like DDM hence they will likely fail to extract MPs from inclusion bodies or similar materials.
CALX-173-GK, a novel glycosylated calixarene detergent, was recently produced consisting of three saccharide groups, a calixarene ring and hydrophobic tail of seven carbon atoms [22] (Figure 2). While CALX-173-GK shows low solubilisation efficacy, ATPase activity of the BmrA transporter reconstituted in DDM/CALX-173-GK was significantly elevated (more than 6-fold) compared with that reconstituted in DDM alone highlighting the potential advantage of supplementing canonical ‘solubilising’ detergents (DDM) with novel ‘stabilising’ detergents [22]. Recently, Agez et al. [23], demonstrated an alternative approach, deploying two calixarene detergents, CALX-R2 (CALX8) and CALX-173-GK, in tandem to efficiently perform the extraction and stabilisation phases of human CD20 solubilisation respectively considering their differing hydrophobicities and thus ability to partition MPs. Reconstitution into CALX-173-GK micelles resulted in stable, homogenous CD20 particles visible through negative stain EM, which did not fuse or aggregate, highlighting its potential application in Cryo-EM [23].
Despite some of the aforementioned detergents showing superior protein stability, in many cases, the issue of poor solubilisation capabilities remains. Consequently, extraction must typically be performed using conventional detergents prior to exchange into these novel detergents. Such exchange steps may be detrimental to MP structure and impact upon their function [5,24–27]. Such intricacies, coupled with the elevated expense associated with novel detergents mean DDM remains the favourable choice of detergent for MP solubilisation accounting for approximately 50% of all unique structures solved between 2010 and 2019 [28]. Increased research interest in MPs will likely necessitate further assessment and characterisation of such novel detergents, elevating their usage and encouraging further optimisation. The pool of available detergents is becoming ever more diverse thus slowly aligning with the complex nature of MPs.
Lipid containing systems
Whilst detergent mediated solubilisation of MPs remains the canonical approach, identification of the optimal detergent can be expensive and time consuming, necessitating consideration of numerous variables [29]. Furthermore, micelles present a relatively poor lipid-bilayer mimetic, generally attributed to the lack of lateral pressure usually implemented by the membrane [30]. Furthermore, neutron reflectometry has revealed the membrane bilayer to be a complex structure with physiochemically distinct layers [31], which detergent micelles cannot replicate comprehensively due to their micellar morphology differing fundamentally from the membrane leaflet architecture. Consequently, MP stability, structure and activity within these mimetic systems may be altered compared with those within the native membrane environment.
To alleviate the limitations associated with the structural/function characterisation of detergent-solubilised MPs, detergent-extracted MPs may be reconstituted into systems themselves containing lipids such as mixed micelles, planar bilayers or liposomes. Of the aforementioned systems, liposomes are particularly important owing to their regular employment during functional investigations of MP transporters or indeed the reconstitution of entire transport systems [32–36], and their ability to partition substrates facilitating assaying. However, despite their ability to more accurately resemble the native lipid environment these techniques often yield heterogeneous populations, in terms of size, composition or protein orientation, which hinder further biochemical studies [37]. Furthermore, that MPs must be initially solubilised in detergent, before subsequent exchange into liposomes, raises the possibility of protein inactivation or degradation.
Nanodiscs
Nanodiscs constitute a discoidal phospholipid bilayer section stabilised by either an amphipathic protein, peptide or polymer which act to shroud the hydrophobic phospholipid tails from the aqueous environment. The first nanodisc systems utilised amphipathic, helical belt proteins derived from human ApoA1, the major proteinaceous component of high-density lipoprotein (HDL) particles, to encapsulate the bilayer section (Figure 3) [38,39]. Genetic engineering of ApoA1 yielded an array of so-called ‘membrane scaffold proteins’ (MSPs) enabling modulation of nanodisc size and incorporation of a range of tags [40,41]. Preliminary modifications yielded MSP1, a construct lacking the N-terminal globular domain of ApoA1, and MSP2, a construct containing two copies of MSP1 joined in a head to tail fashion via a short linker [39]. For a comprehensive list of MSP variants developed subsequently, including their sequence composition, please refer to [41].
Figure 3. Schematic representation of MP incorporation into MSP and polymer nanodiscs.
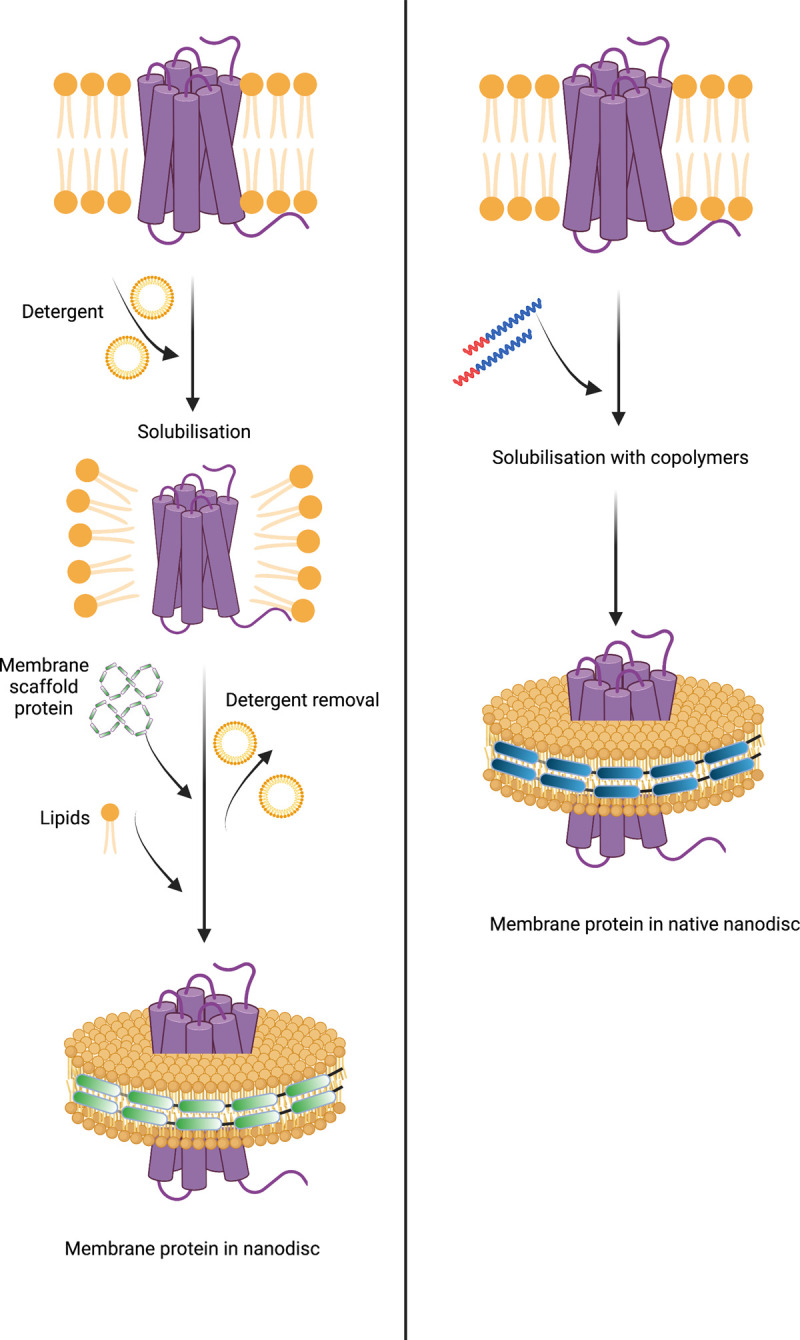
(Left) MSP nanodisc solubilisation — MP is initially extracted into detergent micelles to which lipid and MSP are added before detergent removal triggers nanodisc formation. (Right) Amphipathic polymer nanodisc solubilisation — addition of amphipathic polymer to the membrane fraction results in the spontaneous formation of nanodiscs encapsulating the native lipid and protein. Figure created with BioRender.com.
MSP nanodiscs present an attractive biochemical tool considering their tunable size, lipid composition and tagging capabilities hence their applications are numerous and continue to grow. A sample of MSP nanodisc applications include binding experiments (surface plasmon resonance [42], localised surface plasmon resonance [43]), fractionation methods (electrophoresis [44], ultracentrifugation [45], chromatography [46]), spectroscopic studies [47] and structural determination (NMR [48]), X-ray crystallography [49], electron microscopy [50], small-angle x-ray scattering [51], small-angle neutron scattering [52], analytical ultracentrifugation [53], neutron reflectometry [54] highlighting their diverse potential.
A significant development in nanodisc technology was the utilisation of the styrene maleic acid (SMA) copolymer to form SMA-lipid particles (SMALPs) in 2009 (Table 1) [55]. Such polymer-based nanodiscs display a distinct advantage over their protein stabilised predecessors as they may directly extract proteins from the membrane, negating the need for initial detergent solubilisation, hence lipids contributing to structure or function likely remain associated with the protein following extraction (Figure 3) [56]. To date, SMA solubilisation has been effectively used for a wide range of MPs including ABC-family of transporters [57], ion channels [58,59] and G-protein coupled receptors [60]. However, the incompatibility of SMALPs to low pH (<6.5) and divalent cations (>5 mM) coupled with a tendency to form populations of heterogeneous disc size and interfere with Ni-NTA resin [56,61] severely restricts their usage in structural and functional studies. Consequently, research within the nanodisc field has centred largely upon developing polymers which may be utilised in a wider range of applications than their predecessors as well those possessing novel functionalities.
Table 1. Structure of the amphipathic copolymers discussed in this review along with their respective advantages and disadvantages.
| Polymer | Advantages | Disadvantages |
|---|---|---|
SMA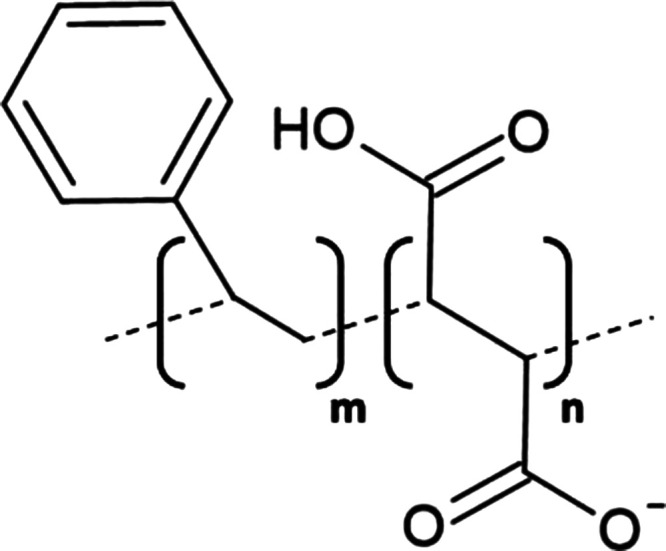
|
Effective solubilisation of a wide range of MPs Retention of native lipid environment Commercially available |
Precipitation at acidic pH (<6.5) Sensitivity to mM concentrations of divalent cations Absorption of UV light (styrene moiety) Nanodisc diameter limited to ∼10 nm May interfere with binding to Ni-NTA resin or other affinity matrices |
SMA-ED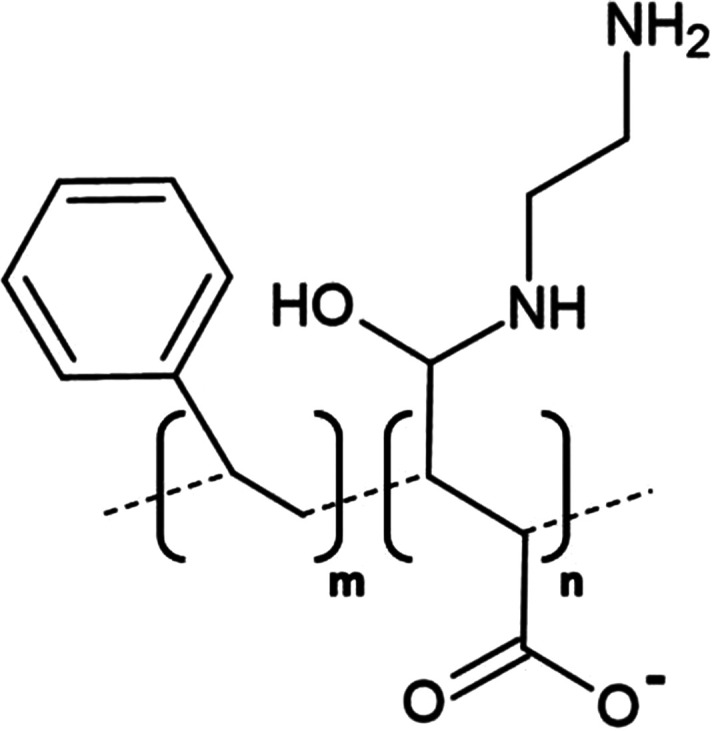
|
Stability over a greater pH range (pH < 5 and pH > 7) Greater tolerance of salt and divalent cations |
Instability between pH 5–7 Absorption of UV light (styrene moiety) Not commercially available |
SMAd-A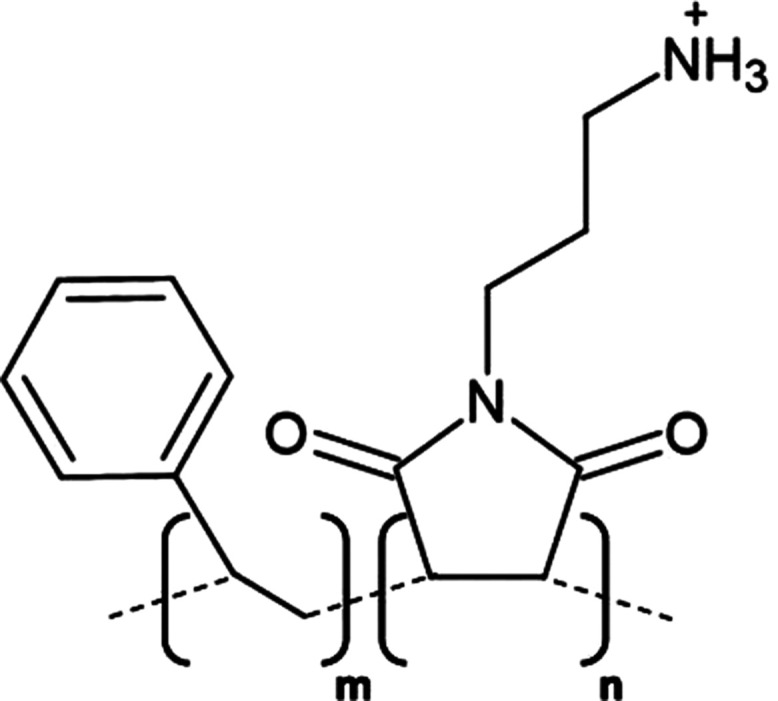
|
Stability under acidic pH (pH < 6) Greater tolerance of salt and divalent cations |
Absorption of UV light (styrene Pmoiety) Precipitation at basic pH (>6) Not commercially available |
SMA-QA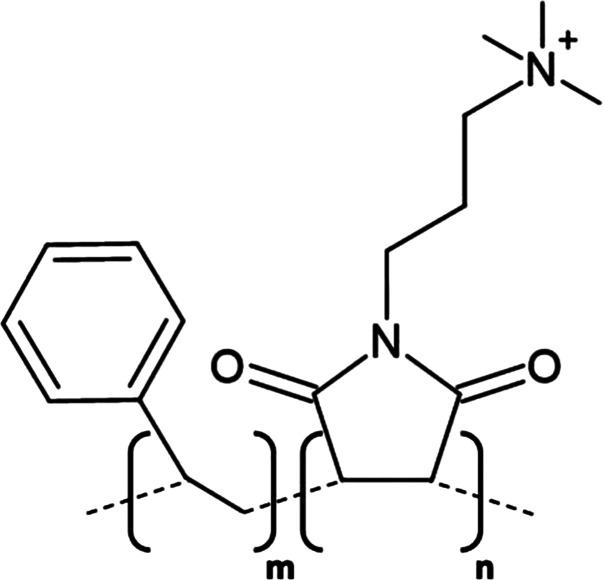
|
Stable over all biologically relevant pH values (2.5 < pH < 10) Ability to modulate nanodisc size including formation of macro-nanodiscs (∼30 nm) Greater tolerance of salt and divalent cations Alignment in the presence of an external magnetic field |
Absorption of UV light (styrene moiety) Not commercially available |
SMA-EA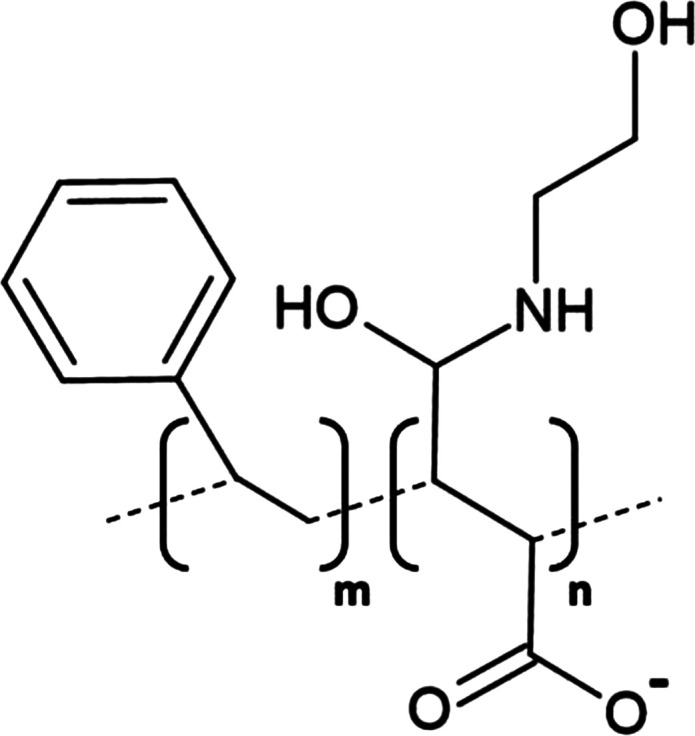
|
Stable under acidic pH values up to pH ∼3.3 Ability to modulate nanodisc size including formation of macro-nanodiscs (∼60 nm) Greater tolerance of salt and divalent cations Improved thermal stability Alignment in the presence of an external magnetic field |
Absorption of UV light (styrene moiety) Not commercially available |
SMI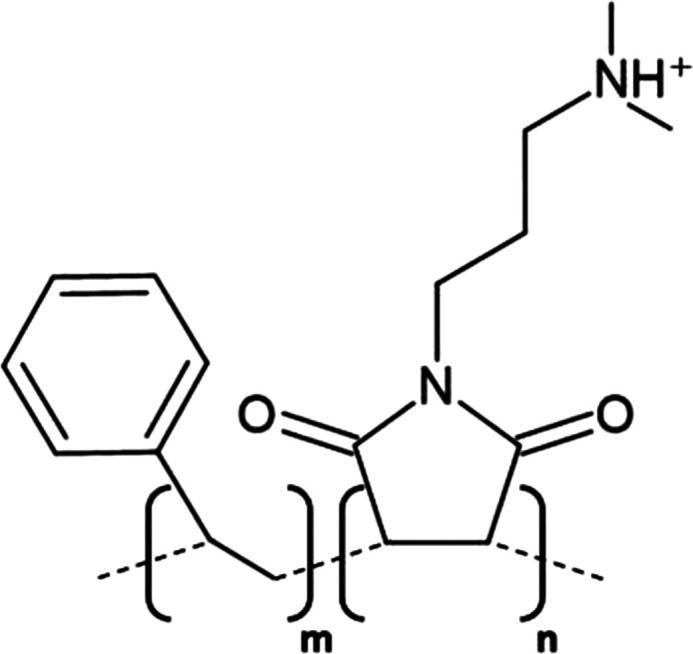
|
Stable over a greater pH range (pH < 7.8) Completely resistant to divalent cations Smaller nanodisc size Improved thermal stability |
Instability at pH > 7.8 Absorption of UV light (styrene moiety) Smaller nanodisc size Possible interactions with biomolecules during the purification process Not commercially available |
SMA-SH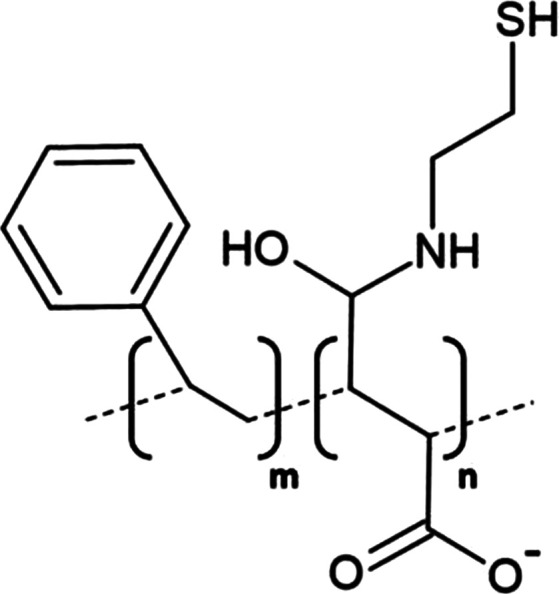
|
Reactive sulfhydryl group allowing conjugation to thiol-reactive compounds | Precipitation at acidic pH (<6.5) Sensitivity to mM concentrations of divalent cations Absorption of UV light (styrene moiety) Not commercially available |
zSMA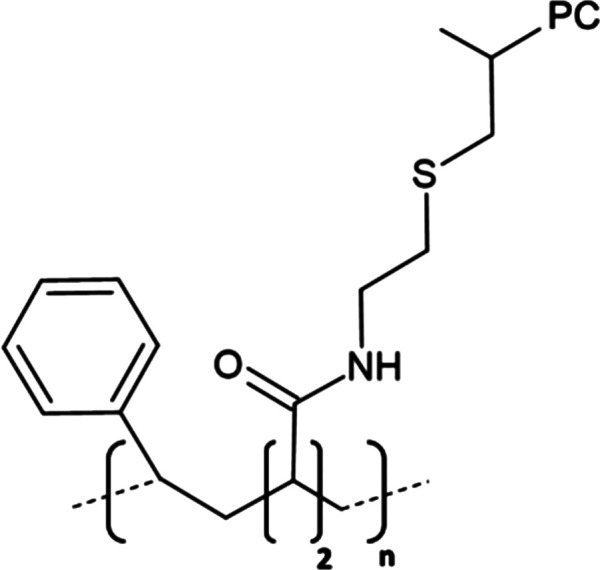
|
Remains soluble at acidic pH values (down to ∼pH 4) Greater tolerance of salt and divalent cations Modulation of nanodisc size |
Absorption of UV light (styrene moiety) Not commercially available |
DIBMA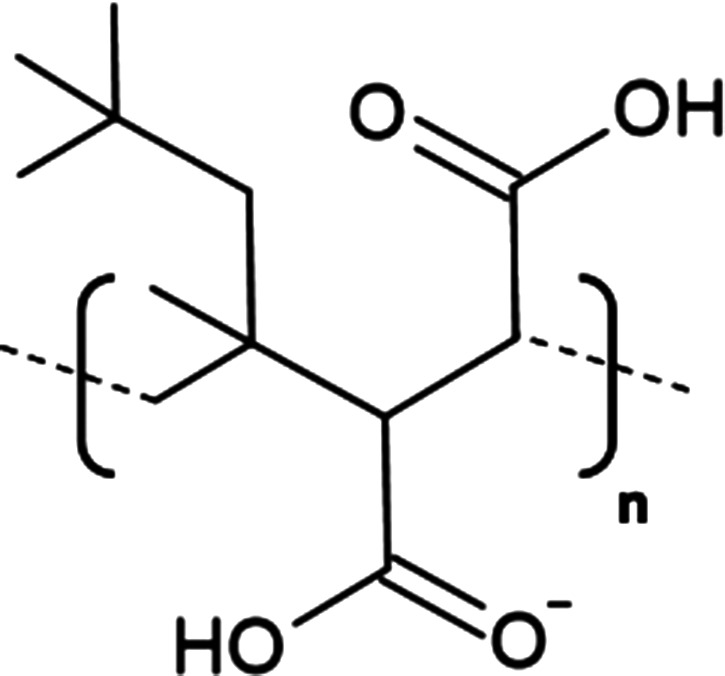
|
Suitable for UV spectroscopic studies (no styrene moiety) Less perturbation of bilayer dynamics within the nanodisc Greater tolerance of salt and divalent cations Larger nanodisc size (∼20 nm) Commercially available |
Precipitation at acidic pH values |
PMA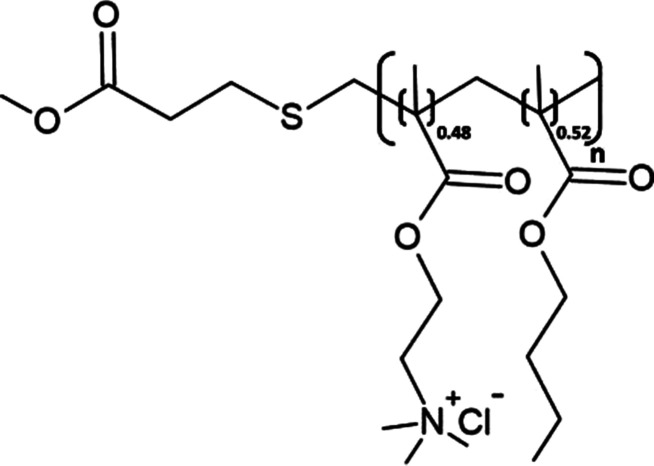
|
Suitable for UV spectroscopic studies (no styrene moiety) Greater tolerance of salt and divalent cations Effective solubilisation under mild conditions Commercially available |
MP solubilisation attributes largely unexplored |
Alkyl-PAAs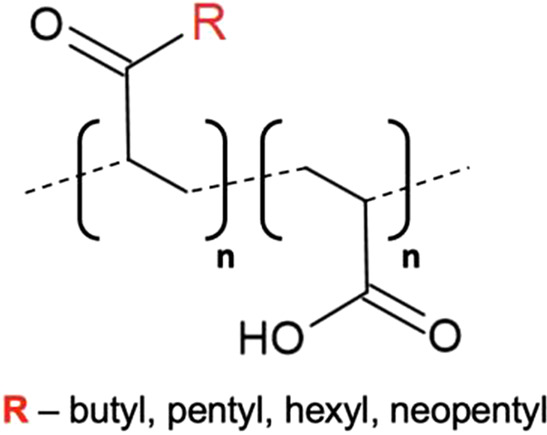
|
Ability to modulate nanodisc size including formation of macro-nanodiscs Suitable for UV spectroscopic studies (no styrene moiety) Alignment in the presence of an external magnetic field Ability to modulate the degree of bilayer perturbation |
Sensitivity to acidic pH and divalent cations MP solubilisation attributes largely unexplored Not commercially available |
AASTY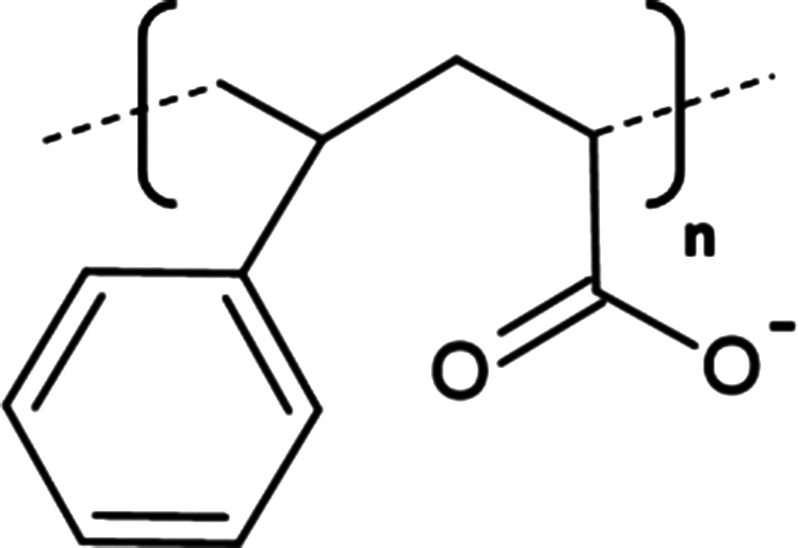
|
Increased extraction efficacy Greater tolerance of salt and divalent cations |
Absorption of UV light (styrene moiety) MP solubilisation attributes largely unexplored Not commercially available |
Structures created with ACD/Labs Chemsketch.
Initial modifications entailed variation of the styrene : maleic acid ratio, to adjust polymer hydrophobicity, however, subsequent functionalisation of the maleic anhydride moiety, of a short, commercially available SMA (1.3 : 1) polymer, has conceived an array of polymers with varied characteristics, many of which address the limitations of SMA (Table 1). Functionalisation of SMA with ethylenediamine yields SMA-ED, a zwitterionic polymer, which may be subsequently dehydrated to yield SMAd-A, itself possessing a primary amine [62]. Both of these species display greater tolerance towards salt and divalent cations (up to 200 mM) and are stable under a greater range of pH values [62]. A quaternary amine containing derivative, SMA-QA, is effective over a range of biologically relevant pH values (2.5–10) thus offers a considerable improvement over SMA in this respect [63,64]. In addition, SMA-QA is capable of forming macro-nanodiscs of ∼30 nm in diameter much like SMA-EA, an ethanolamine containing derivative, which may form macro-nanodiscs up to 60 nm in diameter [63–65]. The ability of both derivatives to form a range of disc sizes including macro-nanodiscs, tunable by polymer:lipid ratio, raises the possibility of their utilisation in electron microscopy whilst their magnetic alignment properties may prove beneficial for NMR. A positively charged copolymer, SMI, displays pH stability inverse to that of SMA, remaining soluble only below pH 7.8, is entirely divalent resistant and forms nanodiscs of a smaller diameter, hence it provides an ideal polymer for the solubilisation of membranes and smaller proteins under acidic pHs [66] (Table 1). SMA-SH, an SMA derivative harbouring a sulfhydryl group, may be conjugated to thiol-reactive compounds, or tethered to functionalised surfaces, hence it presents an ideal candidate for surface-based binding assays (surface plasmon resonance, quartz crystal microbalance with dissipation monitoring) and fluorescence studies (Table 1) [67]. Finally, a new family of zwitterionic SMA copolymers (zSMAs) have been developed by substituting the maleic acid moiety of SMA to maleic amide conjugated to phosphatidylcholine groups, with copolymer length defining individual family members [68] (Table 1). zSMA remains soluble at low pH and/or in the presence of millimolar polyvalent cation concentrations whilst crucially polymer size and zSMA nanodisc diameter are positively correlated hence nanodisc size may be easily modulated [68,69].
Another limitation of SMA is its significant absorption of UV light, due to the styrene moiety, which severely interferes with spectroscopic studies of solubilised proteins [70]. To mitigate this issue, polymers lacking the styrene hydrophobic group have been developed. Diisobutylene maleic acid (DIBMA) is composed of alternating diisobutylene and maleic acid moieties [70] (Table 1). DIBMA forms larger nanodics (12–29 nm), with less ordered lipids, potentially providing a more accommodating environment for certain MPs than SMA [70]. In contrast with SMA, DIBMA displays a higher tolerance to divalent cations, enabling its utilisation in biophysical assays which require their presence, such as ATPase activity [70]. Surprisingly, the presence of low millimolar concentrations of Mg2+ and Ca2+ has been shown to increase the efficacy of DIBMA mediated solubilisation [71]. However, precipitation at acidic pH remains a vulnerability of DIBMA due to retention of the maleic acid carboxyl groups. To date, DIBMA has been successfully implemented in the purification of several MPs, however, when compared directly with SMA, DIBMA nanodiscs typically display reduced stability over time and provide lower protein yields of reduced purity [72].
A poly(methacrylate) (PMA) copolymer was synthesised in 2017 by Yasuhara et al. [73], from hydrophobic butylmethacrylate and cationic methacholine chloride monomers, and has been shown to both solubilise lipid membranes and form nanodiscs (Table 1). PMA is completely styrene and maleic acid free thus this novel polymer provides a promising alternative for biochemical investigation such as circular dichroism or fluorescence-based studies. Despite the anticipated advantages over SMA, only recently have the MP solubilisation attributes of the polymer begun to be assessed. Lavington and Watts [74] successfully performed detergent-free PMA solubilisation of the GPCR neurotensin receptor 1 (NTR1) [74]. PMA displayed solubilisation efficacy comparable to that of conventional detergents in relatively mild solubilisation conditions (26°C pH 7.4–7.6) with no precipitation in the presence of divalent cations thus showing clear advantage over SMA [74]. Such characteristics imply PMA may yet provide a legitimate membrane mimetic for use in biophysical and high-resolution structural studies.
Hydrophobic modification of poly(acrylic acid) (PAA) has previously been shown to yield amphipathic polymers capable of directly extracting MPs from their native membranes [75,76]. Functionalisation of PAA with relatively short alkyl groups (C4–6) has recently been shown to produce a series of amphipathic polymers (alkyl-PAAs) capable of forming nanodiscs, the size of which is dependent upon the lipid : polymer ratio [65]. The tendency of alkyl-PAA macro-nanodiscs to align with a magnetic field suggests a potential application in NMR spectroscopy [65]. Alkyl-PAAs were shown to be capable of extracting MPs directly from the membrane with similar efficacy to SMA [65]. The tolerance of alkyl-PAAs to pH and divalent cations was also shown to be comparable to that of SMA, due to the presence of the carboxylic group, however, the absence of aromatic moieties permits their use in spectroscopic studies [65]. In addition, the length of the functional alkyl chain appeared to directly influence the degree of lipid-bilayer perturbation, with longer alkyl chains generating increased disorder of the encased lipids, suggesting simple modifications may enable modulation of alkyl-PAA solubilisation characteristics [65].
PAA also provided part of the basis for the recently developed poly(acrylic acid-co-styrene) copolymer (AASTY) which comprises styrene and acrylic acid moieties [77]. AASTY has been shown to efficiently extract hTRPM4 from mammalian cells into nanodiscs, with two of the four AASTY polymers assessed displaying extraction efficacies approximately five times that of SMA2000, and display increased stability in the presence of divalent cations [77]. Single-particle cyro-EM of AASTY solubilised hTRPM4 appeared to show promising results, however, the sample exhibited insufficient homogeneity for a high-resolution structure, although this may in part be due to intricacies associated with the specific protein utilised in this study rather than the polymer itself [77]. Considering the favourable aforementioned characteristics of alkyl-PAAs and AASTY, PAA presents a promising foundation for the development of future polymer architectures.
Amphipols
Amphipols (APols) are short amphipathic polymers that self-assemble to conceal hydrophobic MP portions, maintaining their solubility in aqueous solutions, developed to address detergent-associated stumbling blocks in MP research [78] (for comprehensive reviews please refer to [1,79]). APols themselves do not typically solubilise biological membranes [80,81], thus extraction of MPs must be performed using conventional detergents, however, that APols are very weak detergents may facilitate MP stabilisation. APols do not compete efficiently with protein/lipid interactions thus, dissociated lipids are permitted to rebind upon detergent-Apol exchange, enabling MPs to subsist in a more native, stable state.
The most characterised and widely utilised APol to date is polyacrylate-based A8–35 that has been applied in the study of several MPs including bacteriorhodopsin [82] and cytochrome b6f [3]. Despite multiple favourable characteristics, an apparent sensitivity to pH [76] and divalent cations [83] necessitated the development of A8–35 derivatives with modified structures, examples include; non-ionic glycosylated APols (NAPols) [80,84,85], sulfonated APols (SAPols) [86] and phosphorylcholine-based APols (PC-APols) [87]. In addition, APols have been labelled/functionalised to expand their use in MP characterisation including isotopic labelling, the addition of affinity tags and fluorophores (Extensively reviewed in [88]). More recently, Bosco et al. [89], developed biotin functionalised non-ionic APols (BNAPols) that successfully stabilised immobilised functional growth hormone secretagogue receptor (GHSR) upon a streptavidin-coated surface enabling screening of potential ligands via a binding assay.
For single-particle Cryo-EM APols provide an attractive proposition as they eliminate the background noise, often present due to free micelles in detergent-protein samples, and potentially enable proteins to retain co-purifying lipids that may lock them in more stable and or native conformations. APol A8-35 and PMAL-C8 are the most commonly used for determining high-resolution structures with the highest achieved being the bovine-bestrophin-2 anion channel at 2.17 Å [90]. The aptness of APols for Cryo-EM stimulated the development of novel CyclApols which consolidate the properties of SMA and A8–35. Replacing the linear n-alkyl chain of classical A8–35 with cyclic hydrocarbon groups confers the ability of CyclAPols to efficiently solubilise biological membranes [91]. Mimicking this property of SMA eliminates the requirement for classical detergent mediated extraction and subsequent APol exchange that can, in some cases, result in MP destabilisation. A recent preprint reported the use of CyclAPols in the solubiliation of the model bacterial membrane transporter AcrB which subsequently yielded a 3.2 Å Cryo-EM structure [92].
Conclusion
Artificial systems that conceal and stabilise the hydrophobic regions of MPs, allowing their characterisation in aqueous environments, are only epigones of the native lipid environment. Whilst the diversification of MPs is of paramount importance to cellular processes, it also means that the process of liberating and incorporating individual MPs into membrane mimetic systems, without compromising the native structure and/or function, is highly MP-specific. Hence, numerous time consuming, expensive considerations and screening steps are typically employed prior to successfully obtaining a MP isolated in a state suitable for structural/functional investigations.
In the past decade, numerous diverse lipid membrane mimetic systems have been developed, expanding the pool of available solubilisation strategies. Canonical detergents have been further modified, giving rise to an array of innovative structures displaying improved MP stabilisation characteristics. In addition to SMA and its subsequent derivatives, which display increased tolerance to cations and acidic pHs, novel polymers with distinct architectures have been developed. Despite showing initial favourable properties, these polymers require further characterisation to comprehensively assess the appropriateness of their utilisation in MP solubilisation. Finally, a new class CyclAPols, capable of directly extracting MPs from the membrane and maintaining their solubility in aqueous environments, appear a promising candidate for Cryo-EM applications.
Presently, conventional detergents remain the forerunners in MP research owed to their well-defined characteristics, cost and an extensive repertoire of successful applications encompassing high-resolution structures and functional characterisation. However, it remains unequivocal that the ongoing development of membrane mimetic systems is of paramount importance to facilitate future MP characterisation.
Perspectives
Highlight the importance of the field: MP are indispensable components of biological membranes, however, their structural and functional investigation often necessitates their extraction from the heterogeneous membrane environment into a more consistent background. The development of novel and the evolution of existing membrane mimetic systems is, therefore, essential to expand the repertoire of MPs available for scientific and pharmaceutical research.
Summary of the current thinking: The array of membrane mimetic systems available to researchers continues to grow, now encompassing three major categories: Detergents, Nanodiscs and Amphipols. However, solubilisation of most MPs continues to be performed by canonical detergents, particularly maltosides such as DDM, with the more novel systems typically reserved for niche cases, often to accommodate a specific technique, or upon exhaustion of traditional detergent options.
Comment on future directions: The plethora of existing membrane mimetics will likely adequately service the extraction and stabilisation of most MPs, however, those requiring more specialist mimetics will likely increase as research interest focuses upon more complex MPs and their complexes. Increased utilisation of these more novel mimetics, and those yet to be conceived, will assist in their optimisation, characterisation and may result in their adoption as more mainstream techniques. However, it must be acknowledged that classical detergents, particularly maltosides, will likely remain at the forefront of MP solubilisation.
Acknowledgements
We thank Prof. Tim Dafforn and Dr. David Hardy for insightful discussion on the topic of membrane protein solubilisation.
Abbreviations
- AASTY
poly(acrylic acid-co-styrene) CoPolymer
- APols
amphipols
- B2AR
B2 adrenergic receptor
- BNAPols
biotin functionalised non-ionic amphipols
- CMC
critical micelle concentration
- Cryo-EM
cryo-electron microscopy
- CyclApols
amphipols bearing cycloalkane side groups
- DDM
n-dodecyl-β-d-maltoside
- DIBMA
diisobutylene maleic acid copolymer
- Fas
facial amphiphiles
- GHSR
growth hormone secretagogue receptor
- GPCR
G-protein coupled receptor
- HDL
high-density lipoprotein
- LSPR
localised surface plasmon resonance
- MP
membrane protein
- MSP1D1
membrane scaffold protein 1D1
- MSPs
membrane scaffold proteins
- NAPols
non-ionic glycosylated amphipols
- NMR
nuclear magnetic resonance
- NTR1
neurotensin receptor 1
- PAA
poly(acrylic acid)
- PC-APols
phosphorylcholine-based amphipols
- PMA
poly(methacrylate)
- SAPols
sulfonated amphipols
- SMA
styrene maleic acid copolymer
- SMALP
styrene maleic acid lipid particles
- VEG
vitamin-E based glycoside amphiphiles
- zSMAs
zwitterionic SMA
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
We would also like to thank the funding bodies who support us. G.R. and B.F.C. are jointly funded by the BBSRC and the University of Birmingham (through the Midlands Integrative Biosciences Training Partnership — grant no. BB/M01116X/1). G.R., B.F.C. and T.J.K. are also supported by BBSRC Research grant no. BB/S017283/1.
Open Access
Open access for this article was enabled by the participation of University of Birmingham in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
Author Contributions
G.R., B.F.C. and T.J.K. co-wrote the manuscript.
References
- 1.Popot, J.L. (2010) Amphipols, nanodiscs, and fluorinated surfactants: three nonconventional approaches to studying membrane proteins in aqueous solutions. Annu. Rev. Biochem. 79, 737–775 10.1146/annurev.biochem.052208.114057 [DOI] [PubMed] [Google Scholar]
- 2.Helenius, A. and Simons, K. (1975) Solubilization of membranes by detergents. Biochim. Biophys. Acta 415, 29–79 10.1016/0304-4157(75)90016-7 [DOI] [PubMed] [Google Scholar]
- 3.Breyton, C., Tribet, C., Olive, J., Dubacq, J.P. and Popott, J.L. (1997) Dimer to monomer conversion of the cytochrome b6f complex: causes and consequences. J. Biol. Chem. 272, 21892–21900 10.1074/jbc.272.35.21892 [DOI] [PubMed] [Google Scholar]
- 4.Lee, A.G. (2011) Lipid-protein interactions. Biochem. Soc. Trans. 39, 761–766 10.1042/BST0390761 [DOI] [PubMed] [Google Scholar]
- 5.Seddon, A.M., Curnow, P. and Booth, P.J. (2004) Membrane proteins, lipids and detergents: not just a soap opera. Biochim. Biophys. Acta 1666, 105–117 10.1016/j.bbamem.2004.04.011 [DOI] [PubMed] [Google Scholar]
- 6.Ehsan, M., Das, M., Stern, V., Du, Y., Mortensen, J.S., Hariharan, P.et al. (2018) Steroid-based amphiphiles for membrane protein study: the importance of alkyl spacers for protein stability. ChemBioChem 19, 1433–1443 10.1002/cbic.201800106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boussambe, G.N.M., Guillet, P., Mahler, F., Marconnet, A., Vargas, C., Cornut, D.et al. (2018) Fluorinated diglucose detergents for membrane-protein extraction. Methods 147, 84–94 10.1016/j.ymeth.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 8.Wehbie, M., Onyia, K.K., Mahler, F., Le Roy, A., Deletraz, A., Bouchemal, I.et al. (2021) Maltose-based fluorinated surfactants for membrane-protein extraction and stabilization. Langmuir 37, 2111–2122 10.1021/acs.langmuir.0c03214 [DOI] [PubMed] [Google Scholar]
- 9.Das, M., Du, Y., Mortensen, J.S., Bae, H.E., Byrne, B., Loland, C.J.et al. (2018) An engineered lithocholate-based facial amphiphile stabilizes membrane proteins: assessing the impact of detergent customizability on protein stability. Chemistry 24, 9860–9868 10.1002/chem.201801141 [DOI] [PubMed] [Google Scholar]
- 10.Das, M., Du, Y., Mortensen, J.S., Hariharan, P., Lee, H.S., Byrne, B.et al. (2018) Rationally engineered tandem facial amphiphiles for improved membrane protein stabilization efficacy. ChemBioChem 19, 2225–2232 10.1002/cbic.201800388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghani, L., Munk, C.F., Zhang, X., Katsube, S., Du, Y., Cecchetti, C.et al. (2019) 3,5-Triazine-cored maltoside amphiphiles for membrane protein extraction and stabilization. J. Am. Chem. Soc 141, 19677–19687 10.1021/jacs.9b07883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue, D., Xu, T., Wang, H., Wu, M., Yuan, Y., Wang, W.et al. (2019) Disulfide-containing detergents (DCDs) for the structural biology of membrane proteins. Chemistry 25, 11635–11640 10.1002/chem.201903190 [DOI] [PubMed] [Google Scholar]
- 13.Bae, H.E., Du, Y., Hariharan, P., Mortensen, J.S., Kumar, K.K., Ha, B.et al. (2019) Asymmetric maltose neopentyl glycol amphiphiles for a membrane protein study: effect of detergent asymmetricity on protein stability. Chem. Sci. 10, 1107–1116 10.1039/C8SC02560F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Heijne, G. and Gavel, Y. (1988) Topogenic signals in integral membrane proteins. Eur. J. Biochem. 174, 671–678 10.1111/j.1432-1033.1988.tb14150.x [DOI] [PubMed] [Google Scholar]
- 15.von Heijne, G. (1992) Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225, 487–494 10.1016/0022-2836(92)90934-C [DOI] [PubMed] [Google Scholar]
- 16.Suwinska, K., Shkurenko, O., Mbemba, C., Leydier, A., Jebors, S., Coleman, A.W.et al. (2008) Trianionic calix[4]arene monoalkoxy derivatives: synthesis, solid-state structures and self-assembly properties. New J. Chem. 32, 1988–1998 10.1039/b806342g [DOI] [Google Scholar]
- 17.Matar-Merheb, R., Rhimi, M., Leydier, A., Huché, F., Galián, C., Desuzinges-Mandon, E.et al. (2011) Structuring detergents for extracting and stabilizing functional membrane proteins. PLoS One 6, e18036 10.1371/journal.pone.0018036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy, D., Bill, R.M., Jawhari, A. and Rothnie, A.J. (2016) Overcoming bottlenecks in the membrane protein structural biology pipeline. Biochem. Soc. Trans. 44, 838–844 10.1042/BST20160049 [DOI] [PubMed] [Google Scholar]
- 19.Privé, G.G. (2007) Detergents for the stabilization and crystallization of membrane proteins. Methods 41, 388–397 10.1016/j.ymeth.2007.01.007 [DOI] [PubMed] [Google Scholar]
- 20.Otzen, D. (2011) Protein-surfactant interactions: a tale of many states. Biochim. Biophys. Acta 1814, 562–591 10.1016/j.bbapap.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 21.Feroz, H., Kwon, H., Peng, J., Oh, H., Ferlez, B., Baker, C.S.et al. (2018) Improving extraction and post-purification concentration of membrane proteins. Analyst 143, 1378–1386 10.1039/C7AN01470H [DOI] [PubMed] [Google Scholar]
- 22.Dauvergne, J., Desuzinges, E.M., Faugier, C., Igonet, S., Soulié, M., Grousson, D.et al. (2019) Glycosylated amphiphilic calixarene-based detergent for functional stabilization of native membrane proteins. ChemistrySelect 4, 5535 10.1002/slct.201901220 [DOI] [Google Scholar]
- 23.Agez, M., Mandon, E.D., Iwema, T., Gianotti, R., Limani, F., Herter, S.et al. (2019) Biochemical and biophysical characterization of purified native CD20 alone and in complex with rituximab and obinutuzumab. Sci. Rep. 9, 13675 10.1038/s41598-019-50031-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dörr, J.M., Koorengevel, M.C., Schäfer, M., Prokofyev A, V., Scheidelaar, S., Van Der Cruijsen, E.A.W.et al. (2014) Detergent-free isolation, characterization, and functional reconstitution of a tetrameric K+ channel: the power of native nanodiscs. Proc. Natl Acad. Sci. U.S.A. 111, 18607–18612 10.1073/pnas.1416205112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamshad, M., Charlton, J., Lin, Y.P., Routledge, S.J., Bawa, Z., Knowles, T.J.et al. (2015) G-protein coupled receptor solubilization and purification for biophysical analysis and functional studies, in the total absence of detergent. Biosci. Rep. 35, 188 10.1042/BSR20140171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reading, E., Walton, T.A., Liko, I., Marty, M.T., Laganowsky, A., Rees, D.C.et al. (2015) The effect of detergent, temperature, and lipid on the oligomeric state of MscL constructs: insights from mass spectrometry. Chem. Biol. 22, 593–603 10.1016/j.chembiol.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta, K., Donlan, J.A.C., Hopper, J.T.S., Uzdavinys, P., Landreh, M., Struwe, W.B.et al. (2017) The role of interfacial lipids in stabilizing membrane protein oligomers. Nature 541, 421–424 10.1038/nature20820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choy, B.C., Cater, R.J., Mancia, F. and Pryor, E.E. (2021) A 10-year meta-analysis of membrane protein structural biology: detergents, membrane mimetics, and structure determination techniques. Biochim. Biophys. Acta 1863, 183533 10.1016/j.bbamem.2020.183533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Maire, M., Champeil, P. and Møller J, V. (2000) Interaction of membrane proteins and lipids with solubilizing detergents. Biochim. Biophys. Acta 1508, 86–111 10.1016/S0304-4157(00)00010-1 [DOI] [PubMed] [Google Scholar]
- 30.Van Den Brink-Van Der Laan, E., Chupin, V., Killian, J.A. and De Kruijff, B. (2004) Stability of KcsA tetramer depends on membrane lateral pressure. Biochemistry 43, 4240–4250 10.1021/bi036129d [DOI] [PubMed] [Google Scholar]
- 31.Qian, S., Sharma, V.K. and Clifton, L.A. (2020) Understanding the structure and dynamics of complex biomembrane interactions by neutron scattering techniques. Langmuir 36, 15189–15211 10.1021/acs.langmuir.0c02516 [DOI] [PubMed] [Google Scholar]
- 32.Hall, J.L., Sohail, A., Cabrita, E.J., Macdonald, C., Stockner, T., Sitte, H.H.et al. (2020) Saturation transfer difference NMR on the integral trimeric membrane transport protein GltPh determines cooperative substrate binding. Sci. Rep. 10, 16483 10.1038/s41598-020-73443-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherman, D.J., Xie, R., Taylor, R.J., George, A.H., Okuda, S., Foster, P.J.et al. (2018) Lipopolysaccharide is transported to the cell surface by a membrane-Tomembrane protein bridge. Science 359, 798–801 10.1126/science.aar1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto, T., Kawaguchi, K., Watanabe, S., Agustina, R., Ikejima, T., Ikeda, K.et al. (2018) Characterization of human ATP-binding cassette protein subfamily D reconstituted into proteoliposomes. Biochem. Biophys. Res. Commun. 496, 1122–1127 10.1016/j.bbrc.2018.01.153 [DOI] [PubMed] [Google Scholar]
- 35.Xie, R., Taylor, R.J. and Kahne, D. (2018) Outer membrane translocon communicates with inner membrane ATPase to stop lipopolysaccharide transport. J. Am. Chem. Soc. 140, 12691–12694 10.1021/jacs.8b07656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang, X., Chang, S., Qiao, W., Luo, Q., Chen, Y., Jia, Z.et al. (2021) Structural insights into outer membrane asymmetry maintenance in Gram-negative bacteria by MlaFEDB. Nat. Struct. Mol. Biol. 28, 81–91 10.1038/s41594-020-00532-y [DOI] [PubMed] [Google Scholar]
- 37.Cliff, L., Chadda, R. and Robertson, J.L. (2020) Occupancy distributions of membrane proteins in heterogeneous liposome populations. Biochim. Biophys. Acta 1862, 183033 10.1016/j.bbamem.2019.183033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denisov, I.G., Grinkova Y, V., Lazarides, A.A. and Sligar, S.G. (2004) Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. J. Am. Chem Soc. 126, 3477–3487 10.1021/ja0393574 [DOI] [PubMed] [Google Scholar]
- 39.Bayburt, T.H., Grinkova Y, V. and Sligar, S.G. (2002) Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2, 853–856 10.1021/nl025623k [DOI] [Google Scholar]
- 40.Ritchie, T.K., Grinkova Y, V., Bayburt, T.H., Denisov, I.G., Zolnerciks, J.K., Atkins, W.M.et al. (2009) Chapter 11 reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 464, 211–231 10.1016/S0076-6879(09)64011-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denisov, I.G. and Sligar, S.G. (2017) Nanodiscs in membrane biochemistry and biophysics. Chem. Rev. 117, 4669–4713 10.1021/acs.chemrev.6b00690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hörnschemeyer, P., Liss, V., Heermann, R., Jung, K. and Hunke, S. (2016) Interaction analysis of a two-component system using nanodiscs. PLoS One 11, e0149187 10.1371/journal.pone.0149187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das, A., Zhao, J., Schatz, G.C., Sligar, S.G. and Van Duyne, R.P. (2009) Screening of type I and II drug binding to human cytochrome P450-3A4 in nanodiscs by localized surface plasmon resonance spectroscopy. Anal. Chem. 81, 3754–3759 10.1021/ac802612z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Justesen, B.H., Laursen, T., Weber, G., Fuglsang, A.T., Møller, B.L. and Günther Pomorski, T. (2013) Isolation of monodisperse nanodisc-reconstituted membrane proteins using free flow electrophoresis. Anal. Chem. 85, 3497–3500 10.1021/ac4000915 [DOI] [PubMed] [Google Scholar]
- 45.Ding, Y., Fujimoto, L.M., Yao, Y. and Marassi, F.M. (2015) Solid-state NMR of the Yersinia pestis outer membrane protein ail in lipid bilayer nanodiscs sedimented by ultracentrifugation. J. Biomol. NMR 61, 275–286 10.1007/s10858-014-9893-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansen, N.T., Tidemand, F.G., Nguyen, T.T.T.N., Rand, K.D., Pedersen, M.C. and Arleth, L. (2019) Circularized and solubility-enhanced MSPs facilitate simple and high-yield production of stable nanodiscs for studies of membrane proteins in solution. FEBS J. 286, 1734–1751 10.1111/febs.14766 [DOI] [PubMed] [Google Scholar]
- 47.Ganapathy, S., Opdam, L., Hontani, Y., Frehan, S., Chen, Q., Hellingwerf, K.J.et al. (2020) Membrane matters: the impact of a nanodisc-bilayer or a detergent microenvironment on the properties of two eubacterial rhodopsins. Biochim. Biophys. Acta 1862, 183113 10.1016/j.bbamem.2019.183113 [DOI] [PubMed] [Google Scholar]
- 48.Shenkarev, Z.O., Lyukmanova, E.N., Paramonov, A.S., Panteleev P, V., Balandin S, V., Shulepko, M.A.et al. (2014) Lipid-protein nanodiscs offer new perspectives for structural and functional studies of water-soluble membrane-active peptides. Acta Nat. 6, 84–94 10.32607/20758251-2014-6-2-84-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nikolaev, M., Round, E., Gushchin, I., Polovinkin, V., Balandin, T., Kuzmichev, P.et al. (2017) Integral membrane proteins can be crystallized directly from nanodiscs. Cryst. Growth Des. 17, 945–948 10.1021/acs.cgd.6b01631 [DOI] [Google Scholar]
- 50.Coudray, N., Isom, G.L., Macrae, M.R., Saiduddin, M.N., Bhabha, G. and Ekiert, D.C. (2020) Structure of bacterial phospholipid transporter MlaFEDB with substrate bound. eLife 9, 1–73 10.7554/eLife.62518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skar-Gislinge, N., Kynde, S.A.R., Denisov, I.G., Ye, X., Lenov, I., Sligar, S.G.et al. (2015) Small-angle scattering determination of the shape and localization of human cytochrome P450 embedded in a phospholipid nanodisc environment. Acta Crystallogr. D Biol. Crystallogr. 71(Pt 12), 2412–2421 10.1107/S1399004715018702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Josts, I., Nitsche, J., Maric, S., Mertens, H.D., Moulin, M., Haertlein, M.et al. (2018) Conformational states of ABC transporter MsbA in a lipid environment investigated by small-angle scattering using stealth carrier nanodiscs. Structure 26, 1072–1079.e4 10.1016/j.str.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 53.Xu, H., Hill, J.J., Michelsen, K., Yamane, H., Kurzeja, R.J.M., Tam, T.et al. (2015) Characterization of the direct interaction between KcsA-Kv1.3 and its inhibitors. Biochim. Biophys. Acta 1848, 1974–1980 10.1016/j.bbamem.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 54.Wadsäter, M., Laursen, T., Singha, A., Hatzakis, N.S., Stamou, D., Barker, R.et al. (2012) Monitoring shifts in the conformation equilibrium of the membrane protein cytochrome P450 reductase (POR) in nanodiscs. J. Biol. Chem. 287, 34596–34603 10.1074/jbc.M112.400085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knowles, T.J., Finka, R., Smith, C., Lin, Y.P., Dafforn, T. and Overduin, M. (2009) Membrane proteins solubilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. J. Am. Chem. Soc. 131, 7484–7485 10.1021/ja810046q [DOI] [PubMed] [Google Scholar]
- 56.Pollock, N.L., Lee, S.C., Patel, J.H., Gulamhussein, A.A. and Rothnie, A.J. (2018) Structure and function of membrane proteins encapsulated in a polymer-bound lipid bilayer. Biochim. Biophys. Acta 1860, 809–817 10.1016/j.bbamem.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 57.Gulati, S., Jamshad, M., Knowles, T.J., Morrison, K.A., Downing, R., Cant, N.et al. (2014) Detergent-free purification of ABC (ATP-binding-cassette) transporters. Biochem. J. 461, 269–278 10.1042/BJ20131477 [DOI] [PubMed] [Google Scholar]
- 58.Karlova, M.G., Voskoboynikova, N., Gluhov, G.S., Abramochkin, D., Malak, O.A., Mulkidzhanyan, A.et al. (2019) Detergent-free solubilization of human Kv channels expressed in mammalian cells. Chem. Phys. Lipids 219, 50–57 10.1016/j.chemphyslip.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 59.Yoder, N. and Gouaux, E. (2020) The his-gly motif of acid-sensing ion channels resides in a reentrant ‘loop’ implicated in gating and ion selectivity. eLife 9, e56527 10.7554/eLife.56527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Logez, C., Damian, M., Legros, C., Dupré, C., Guéry, M., Mary, S.et al. (2016) Detergent-free isolation of functional G protein-coupled receptors into nanometric lipid particles. Biochemistry 55, 38–48 10.1021/acs.biochem.5b01040 [DOI] [PubMed] [Google Scholar]
- 61.Broecker, J., Eger, B.T. and Ernst, O.P. (2017) Crystallogenesis of membrane proteins mediated by polymer-bounded lipid nanodiscs. Structure 25, 384–392 10.1016/j.str.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 62.Ravula, T., Hardin, N.Z., Ramadugu, S.K. and Ramamoorthy, A. (2017) PH tunable and divalent metal ion tolerant polymer lipid nanodiscs. Langmuir 33, 10655–10662 10.1021/acs.langmuir.7b02887 [DOI] [PubMed] [Google Scholar]
- 63.Ravula, T., Hardin, N.Z., Ramadugu, S.K., Cox, S.J. and Ramamoorthy, A. (2018) Formation of pH-resistant monodispersed polymer-lipid nanodiscs. Angew. Chem. Int. Ed. 57, 1342–1345 10.1002/anie.201712017 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 64.Ravula, T., Hardin, N.Z., Di Mauro, G.M. and Ramamoorthy, A. (2018) Styrene maleic acid derivates to enhance the applications of bio-inspired polymer based lipid-nanodiscs. Eur. Polym. J. 108, 597–602 10.1016/j.eurpolymj.2018.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hardin, N.Z., Ravula, T., Di, M.G. and Ramamoorthy, A. (2019) Hydrophobic functionalization of polyacrylic acid as a versatile platform for the development of polymer lipid nanodisks. Small 15, 1804813 10.1002/smll.201804813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall, S.C.L., Tognoloni, C., Charlton, J., Bragginton, É.C, Rothnie, A.J., Sridhar, P.et al. (2018) An acid-compatible co-polymer for the solubilization of membranes and proteins into lipid bilayer-containing nanoparticles. Nanoscale 10, 10609–10619 10.1039/C8NR01322E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindhoud, S., Carvalho, V., Pronk, J.W. and Aubin-Tam, M.E. (2016) SMA-SH: modified styrene-maleic acid copolymer for functionalization of lipid nanodiscs. Biomacromolecules 17, 1516–1522 10.1021/acs.biomac.6b00140 [DOI] [PubMed] [Google Scholar]
- 68.Fiori, M.C., Jiang, Y., Altenberg, G.A. and Liang, H. (2017) Polymer-encased nanodiscs with improved buffer compatibility. Sci. Rep. 7, 1–10 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiori, M.C., Zheng, W., Kamilar, E., Simiyu, G., Altenberg, G.A. and Liang, H. (2020) Extraction and reconstitution of membrane proteins into lipid nanodiscs encased by zwitterionic styrene-maleic amide copolymers. Sci. Rep 10, 1–13 10.1038/s41598-020-66852-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oluwole, A.O., Danielczak, B., Meister, A., Babalola, J.O., Vargas, C. and Keller, S. (2017) Solubilization of membrane proteins into functional lipid-bilayer nanodiscs using a diisobutylene/maleic acid copolymer. Angew Chem. Int. Ed. Engl. 56, 1919–1924 10.1002/anie.201610778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Danielczak, B., Meister, A. and Keller, S. (2019) Influence of Mg2+ and Ca2+ on nanodisc formation by diisobutylene/maleic acid (DIBMA) copolymer. Chem. Phys. Lipids 221, 30–38 10.1016/j.chemphyslip.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 72.Gulamhussein, A.A., Uddin, R., Tighe, B.J., Poyner, D.R. and Rothnie, A.J. (2020) A comparison of SMA (styrene maleic acid) and DIBMA (di-isobutylene maleic acid) for membrane protein purification. Biochim. Biophys. Acta 1862, 183281 10.1016/j.bbamem.2020.183281 [DOI] [PubMed] [Google Scholar]
- 73.Yasuhara, K., Arakida, J., Ravula, T., Ramadugu, S.K., Sahoo, B., Kikuchi, J.I.et al. (2017) Spontaneous lipid nanodisc fomation by amphiphilic polymethacrylate copolymers. J. Am. Chem. Soc. 139, 18657–18663 10.1021/jacs.7b10591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lavington, S. and Watts, A. (2021) Detergent-free solubilisation & purification of a G protein coupled receptor using a polymethacrylate polymer. Biochim. Biophys. Acta Biomembr. 1863, 183441 10.1016/j.bbamem.2020.183441 [DOI] [PubMed] [Google Scholar]
- 75.Vial, F., Oukhaled, A.G., Auvray, L. and Tribet, C. (2007) Long-living channels of well defined radius opened in lipid bilayers by polydisperse, hydrophobically-modified polyacrylic acids. Soft Matter. 3, 75–78 10.1039/B613003H [DOI] [PubMed] [Google Scholar]
- 76.Gohon, Y., Giusti, F., Prata, C., Charvolin, D., Timmins, P., Ebel, C.et al. (2006) Well-defined nanoparticles formed by hydrophobic assembly of a short and polydisperse random terpolymer, amphipol A8-35. Langmuir 22, 1281–1290 10.1021/la052243g [DOI] [PubMed] [Google Scholar]
- 77.Smith, A.A.A., Autzen, H.E., Faust, B., Spakowitz, A.J., Cheng, Y. and Appel, E.A. (2020) Lipid nanodiscs via ordered copolymers. Chem 6, 2782–2795 10.1016/j.chempr.2020.08.004 [DOI] [Google Scholar]
- 78.Tribet, C., Audebert, R. and Popot, J.L. (1996) Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc. Natl Acad. Sci. U.S.A. 93, 15047–15050 10.1073/pnas.93.26.15047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zoonens, M. and Popot, J.L. (2014) Amphipols for each season. J. Membr. Biol. 247, 759–796 10.1007/s00232-014-9666-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bazzacco, P., Sharma, K.S., Durand, G., Giusti, F., Ebel, C., Popot, J.L.et al. (2009) Trapping and stabilization of integral membrane proteins by hydrophobically grafted glucose-based telomers. Biomacromolecules 10, 3317–3326 10.1021/bm900938w [DOI] [PubMed] [Google Scholar]
- 81.Champeil, P., Menguy, T., Tribet, C., Popot, J.L. and Le Maire, M. (2000) Interaction of amphipols with sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 275, 18623–18637 10.1074/jbc.M000470200 [DOI] [PubMed] [Google Scholar]
- 82.Gohon, Y., Dahmane, T., Ruigrok, R.W.H., Schuck, P., Charvolin, D., Rappaport, F.et al. (2008) Bacteriorhodopsin/amphipol complexes: structural and functional properties. Biophys. J. 94, 3523–3537 10.1529/biophysj.107.121848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Picard, M., Dahmane, T., Garrigos, M., Gauron, C., Giusti, F., Le Maire, M.et al. (2006) Protective and inhibitory effects of various types of amphipols on the Ca2+-ATPase from sarcoplasmic reticulum: a comparative study. Biochemistry 45, 1861–1869 10.1021/bi051954a [DOI] [PubMed] [Google Scholar]
- 84.Sharma, K.S., Durand, G., Giusti, F., Olivier, B., Fabiano, A.S., Bazzacco, P.et al. (2008) Glucose-based amphiphilic telomers designed to keep membrane proteins soluble in aqueous solutions: synthesis and physicochemical. Langmuir 24, 13581–13590 10.1021/la8023056 [DOI] [PubMed] [Google Scholar]
- 85.Sharma, K.S., Durand, G., Gabel, F., Bazzacco, P., Le Bon, C., Billon-Denis, E.et al. (2012) Non-ionic amphiphilic homopolymers: synthesis, solution properties, and biochemical validation. Langmuir 28, 4625–4639 10.1021/la205026r [DOI] [PubMed] [Google Scholar]
- 86.Dahmane, T., Giusti, F., Catoire, L.J. and Popot, J.L. (2011) Sulfonated amphipols: synthesis, properties, and applications. Biopolymers 95, 811–823 10.1002/bip.21683 [DOI] [PubMed] [Google Scholar]
- 87.Diab, C., Tribet, C., Gohon, Y., Popot, J.L. and Winnik, F.M. (2007) Complexation of integral membrane proteins by phosphorylcholine-based amphipols. Biochim. Biophys. Acta 1768, 2737–2747 10.1016/j.bbamem.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 88.Le Bon, C., Popot, J.L. and Giusti, F. (2014) Labeling and functionalizing amphipols for biological applications. J. Membr. Biol. 247, 797–814 10.1007/s00232-014-9655-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bosco, M., Damian, M., Chauhan, V., Roche, M., Guillet, P., Fehrentz, J.A.et al. (2020) Biotinylated non-ionic amphipols for GPCR ligands screening. Methods 180, 69–78 10.1016/j.ymeth.2020.06.001 [DOI] [PubMed] [Google Scholar]
- 90.Owji, A.P., Zhao, Q., Ji, C., Kittredge, A., Hopiavuori, A., Fu, Z.et al. (2020) Structural and functional characterization of the bestrophin-2 anion channel. Nat. Struct. Mol. Biol. 27, 382–391 10.1038/s41594-020-0402-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marconnet, A., Michon, B., Le Bon, C., Giusti, F., Tribet, C. and Zoonens, M. (2020) Solubilization and stabilization of membrane proteins by cycloalkane-modified amphiphilic polymers. Biomacromolecules 21, 3459–3467 10.1021/acs.biomac.0c00929 [DOI] [PubMed] [Google Scholar]
- 92.Higgins, A., Flynn, A., Marconnet, A., Musgrove, L., Postis, V., Lippiat, J.et al. (2021) Cycloalkane-modied amphiphilic polymers provide direct extraction of membrane proteins for CryoEM analysis. 1–14 10.21203/rs.3.rs-131488/v1 [DOI]