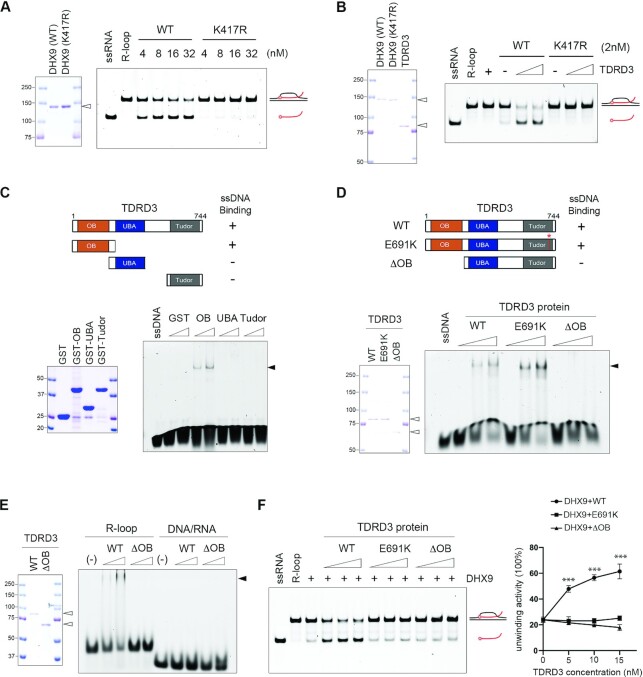
Figure 5.
TDRD3 promotes DHX9 helicase activity in R-loop resolution. (A) DHX9 resolves R-loops in a helicase activity-dependent manner. Coomassie Blue staining of recombinant wild type (WT) and helicase activity-deficient (K417R) DHX9 purified from HEK293 cells (left). The helicase assay on R-loops was performed by incubating increasing amounts of recombinant WT or K417R DHX9 with 5′ 6-FAM-labeled R-loop substrates (5 nM) at 37°C for 10 min. The reaction products were analyzed by gel electrophoresis and fluorescence imaging (right). The open triangle indicates the recombinant proteins. (B) TDRD3 stimulates the helicase activity of WT DHX9, but not K417R mutant DHX9, in R-loop resolution. Coomassie Blue staining of recombinant WT, K417R mutant DHX9, and TDRD3 purified from HEK293 cells (left). The helicase assay on R-loops was performed by incubating 5′ 6-FAM-labeled R-loop substrates with constant amounts of either WT or K417R mutant DHX9 (2 nM) and increasing amounts (40 and 60 nM) of TDRD3 (right). The open triangles indicate the recombinant proteins. (C) The OB-fold of TDRD3 binds single-stranded DNA (ssDNA). A graphic summary of the interactions of truncated TDRD3 fragments with ssDNA is shown (upper panel). Coomassie Blue staining of recombinant GST-fusion proteins of TDRD3, including its OB-fold, UBA domain, and Tudor domain, purified from E. coli (lower left panel). The electrophoretic mobility shift assay (EMSA) was performed by incubating a 5′ 6-FAM-labeled ssDNA oligonucleotide (5 nM) with increasing amounts (25 and 50 nM) of the recombinant TDRD3 proteins (lower right panel). The solid triangle indicates the protein–nucleotide complex. (D) The OB-fold is essential for the interaction of TDRD3 with ssDNA. A graphic summary of the WT, methylarginine binding-deficient (E691K), and OB-fold-truncated (ΔOB) TDRD3 interaction with ssDNA is shown (upper panel). Coomassie Blue staining of all three recombinant TDRD3 proteins purified from HEK293 cells (lower left panel). EMSA was performed to detect the binding of increasing amounts (20, 40 and 60 nM) of recombinant proteins with a 5′ 6-FAM-labeled ssDNA oligonucleotide (5 nM) (lower right panel). The open triangle indicates the recombinant proteins. The solid triangle indicates the protein-nucleotide complex. (E) TDRD3 interacts with R-loops, but not DNA/RNA hybrids. EMSA was performed by incubating increasing amounts (40 and 60 nM) of recombinant WT and OB-fold-truncated (ΔOB) TDRD3 with 5′ 6-FAM-labeled R-loop or DNA/RNA hybrid oligonucleotide (5 nM). The open triangle indicates the recombinant proteins. The solid triangle indicates the protein-nucleotide complex. (F) Both the OB-fold and the functional Tudor domain are required for TDRD3 to stimulate the helicase activity of DHX9 in R-loop resolution. The helicase assay on R-loops was performed by incubating 5′ 6-FAM-labeled R-loop substrates (5 nM) with a constant amount of DHX9 (2 nM) and increasing amounts (20, 40 and 60 nM) of WT, methylarginine binding-deficient (E691K), and ssDNA binding-deficient (ΔOB) TDRD3 (left). Helicase activity was quantified by measuring the percentage of unwound substrates under the indicated assay conditions (right). Statistical analysis was performed using Student's t-tests of data from three independent experiments. *** P < 0.001.