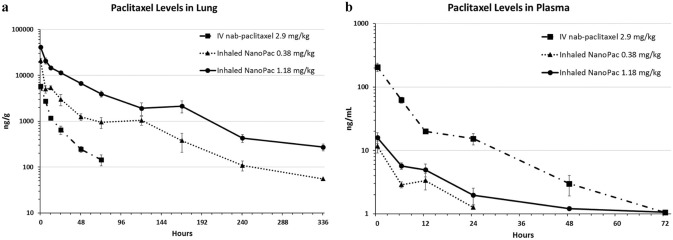
Fig. 4.
Paclitaxel levels in lung (a) and plasma (b) after inhaled NanoPac treatment. Male Sprague–Dawley rats (6–8 weeks old) were administered paclitaxel on a single occasion in one of three treatment arms (n = 30 each): inhaled submicron particle paclitaxel in a nose-only exposure at a low-dose of 0.38 mg/kg or a high dose of 1.18 mg/kg, or intravenous nab-paclitaxel administered via tail vein injection at 2.9 mg/kg. Three animals from each arm were sacrificed at 0.5, 6, 12, 24, 48, 72, 120, 168, 240, and 336 h post exposure for lung tissue and plasma collections. Lung tissue (a) and plasma (b) were assayed via ultra-performance liquid chromatography tandem mass spectrometry to quantify paclitaxel concentration as a function of time with a lower level of quantification of 50 ng/g and 1 ng/mL, respectively (mean ± 1SEM) [6]. Reprinted by permission from Mary Ann Liebert, Inc.: [Mary Ann Liebert] [Journal of Aersol Medicine and Pulmonary] [Pharmacokinetic profile of inhaled submicron particle paclitaxel (NanoPac) in a rodent model, James Verco et al.] [2019]