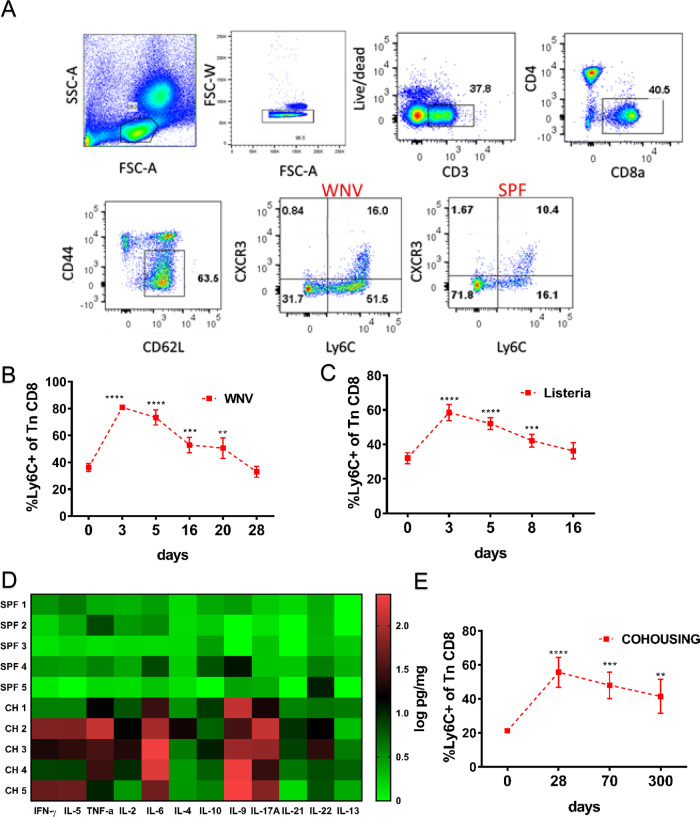
Fig. 2. Naïve CD8+ T cells upregulate Ly6C transiently during acute viral and bacterial infection and permanently under ‘non-SPF’ conditions.
A Flow cytometry gating strategy to analyze the expression of Ly6C and CXC3 on naïve (CD44loCD62Lhi) CD8+ T cells Briefly, live CD3+CD8+ cells were gated with the standard phenotypic definition for Tn cells (CD62LhiCD44lo) and then divided into the positive and negative population by Ly6C expression. B C57BL/6 mice were infected with 1000 PFU WNV virus by footpad injection. Mice were bled retro-orbitally at days 3, 5, 16, 20, and 28 post-infection. Blood was hypotonicially lysed and surface stained with antibodies for flow cytometric analysis. (n = 5 mice per group, data presented as mean ± sd, **p = 0.0049, ***p < 0.001, ****p < 0.0001) C Mice were inoculated intravenously (i.v.) with 104 CFU Listeria monocytogenes. Mice were bled retro-orbitally at days 3, 5, 8, and 16 p.i. The proportion of Ly6C+ positive CD44loCD62Lhi cells were analyzed by flow cytometry (n = 5 mice per group, data presented as mean ± sd, ***p < 0.001, ****p < 0.0001). D Expression of various inflammatory cytokines was measured in homogenates of secondary lymphoid tissue of cohoused and SPF control mice by Legendplex flow cytometry multiplex immunoassay (n = 5 mice per group) (E) cohoused mice were bled periodically and proportion of Ly6C+ positive naïve CD8+s was measured by flow cytometry (n = 5 mice per group, data presented as mean ± sd, **p = 0.0022, ***p < 0.001, ****p < 0.0001). A–E Data are representative of two independent experiments (with n = 5 mice per group) (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). B–E unpaired one-way ANOVA with Dunett’s correction for multiple comparisons.