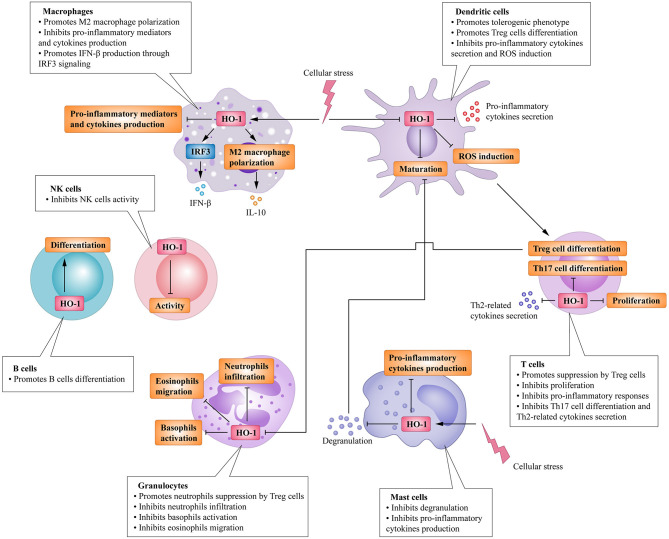
Figure 1.
Immunomodulatory activity of heme oxygenase 1 (HO-1) in immune cells. HO-1 is stress-inducible by macrophages and promotes the phenotypic shift to M2 macrophages, associated anti-inflammatory activities. HO-1 also modulates the production of interferon (IFN)-β in macrophages through direct HO-1 binding to interferon regulatory factor 3 (IRF3) in response to pro-inflammatory stimuli. HO-1 induction in dendritic cells (DCs) inhibits their maturation, secretion of pro-inflammatory cytokines, and induction of ROS. Mast cells (MCs) can sense local stressor insults to induce HO-1, thereby suppressing their degranulation and production of inflammatory cytokines. Moreover, inhibiting MCs' degranulation can also inhibit DCs' maturation. These effects all result in the promotion of DCs into a tolerogenic phenotype, thus promoting regulatory T cell (Treg cell) differentiation. HO-1 expression by T cells can additionally inhibit T helper 17 cells (TH17 cells) differentiation, Th2-related cytokines secretion, as well as limit proliferation. Activated Treg cells can initiate HO-1 expression in neutrophils shifting them to a suppressive phenotype, which inhibits their infiltration, basophil activation as well as migration of eosinophils. HO-1 expression by B cells can additionally promote their development and growth. HO-1 induction can also inhibit NK cells effector functions and activity.