FIGURE.
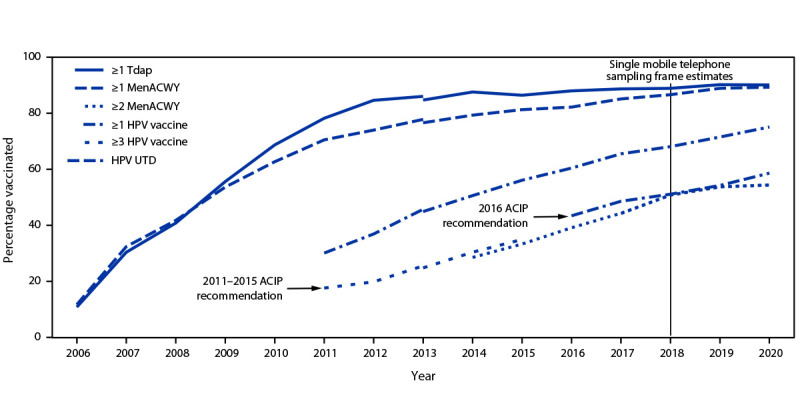
Estimated vaccination coverage with selected vaccines and doses* among adolescents aged 13–17 years, by survey year† — National Immunization Survey–Teen,§,¶ United States, 2006–2020
Abbreviations: ACIP = Advisory Committee on Immunization Practices; HPV = human papillomavirus; MenACWY = quadrivalent meningococcal conjugate vaccine; NIS-teen = National Immunization Survey–Teen; Tdap = tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine; UTD = up to date.
* ≥1 dose Tdap at age ≥10 years; ≥1 dose MenACWY or meningococcal-unknown type vaccine; ≥2 doses MenACWY or meningococcal-unknown type vaccine, calculated only among adolescents aged 17 years at time of interview. Does not include adolescents who received their first and only dose of MenACWY at age ≥16 years; HPV vaccine, nine-valent (9vHPV), quadrivalent (4vHPV), or bivalent (2vHPV). The routine ACIP recommendation for HPV vaccination was made for females in 2006 and for males in 2011. Because HPV vaccination was recommended for males in 2011, coverage for all adolescents was not measured before that year; HPV UTD includes those with ≥3 doses, and those with 2 doses when the first HPV vaccine dose was initiated at age <15 years and at least 5 months minus 4 days elapsed between the first and second dose.
† NIS-Teen implemented a revised adequate provider data definition in 2014 and retrospectively applied the revised definition to 2013 data. Estimates using a revised definition might not be directly comparable.
§ NIS-Teen moved in 2018 to a single-sample frame.
¶ ACIP revised the recommended HPV vaccination schedule in late 2016. The schedule changed from a 3-dose to 2-dose series with appropriate spacing between receipt of the first and second dose for immunocompetent adolescents initiating the series before the 15th birthday. Three doses are still recommended for adolescents initiating the series at age ≥15 years. Because of the change in definition, the graph includes estimates for ≥3 doses of HPV during 2011–2015 and the HPV UTD estimate for 2016–2020. Because HPV vaccination was recommended for males in 2011, coverage for all adolescents was not measured before that year.
