Abstract
Microplastics (MPs) and nanoplastics (NPs) have gained much attention in recent years because of their ubiquitous presence, which is the widely acknowledged threat to the environment. MPs can be <5 mm size, while NPs are <100 nm, and both can be detected in various forms and shapes in the environment to alleviate their harmful effects on aquatic species, soil organisms, birds, and humans. In efforts to address these issues, the present review discusses about sampling methods for water, sediments, and biota along with their merits and demerits. Various identification techniques such as FTIR, Raman, ToF-SIMS, MALDI TOF MS, and ICP-MS are critically discussed. The detrimental effects caused by MPs and NPs are discussed critically along with the efficient and cost-effective treatment processes including membrane technologies in order to remove plastics particles from various sources to mitigate their environmental pollution and risk assessment.
Keywords: Microplastics, Nanoplastics, Sampling, Identification, ToF-SIMS, Ultrafiltration, Membrane technology
1. Introduction
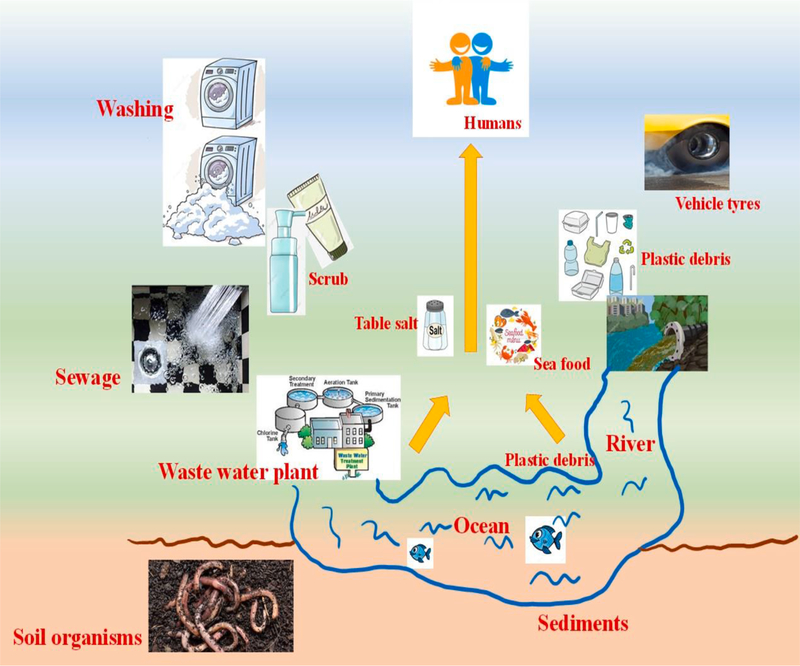
In the present-day scenario, life on the mother earth without the use of plastics is almost impossible as these are the integral part and partial of human life, pervading all corners of society as they are multifaceted, durable, and extensively used materials having numerous applications [1]. The advantages of plastics, including their flexibility, durability, and light resistance have attracted their applications in a wide variety of areas [2]. However, the global population explosion over the years, along with urbanization [2–6] has drastically affected the environment creating numerous environmental and social problems such as increased energy demands, pollution [7], congestion, global warming [8–15], affecting the oceanic, biological and human systems. Plastic particles are found in soil, coastal lines, rivers, remote locations, and different oceans (including Antarctica) due to long-distance transportation of particles through air and river streams. These particles pose high risks to the environment, earth, human health, and integrity of the natural food chains. According to the National Geographic, over 5 trillion pieces of plastic debris are present in the ocean, of which mass of over 300,000 tons float on the surface of the oceans, while over four billion plastics per square kilometre in the deep ocean are present. Year after year, the use of plastics increases, amounting to the worldwide plastics production of nearly 320 million tons per year [16].
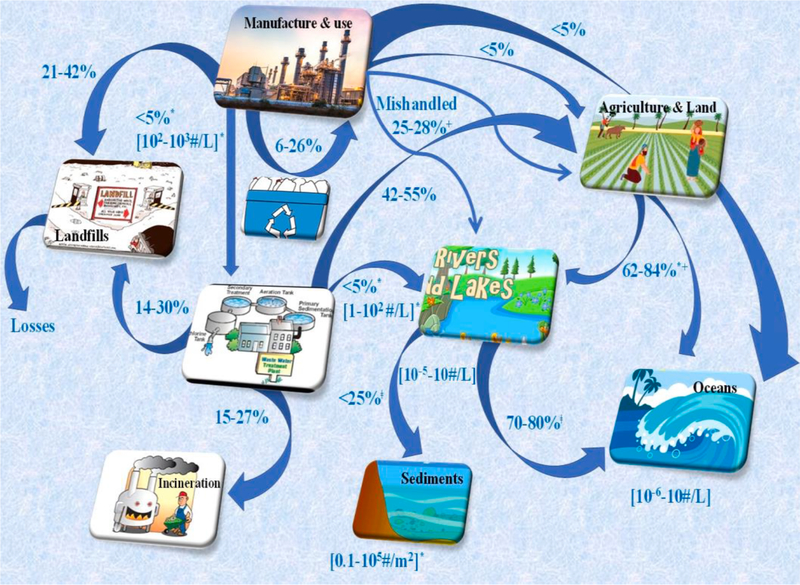
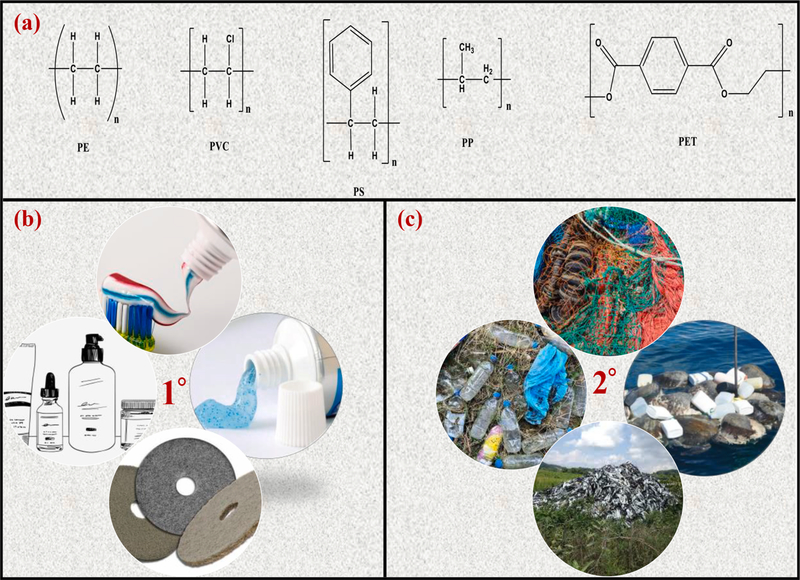
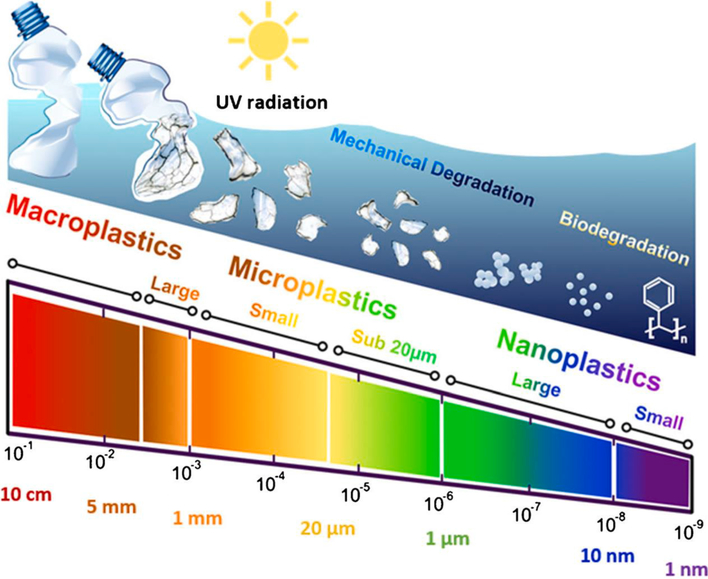
Nearly 100 years back in the year 1920, Herman Staudinger first introduced concept of polymers and proposed for the first time the idea that polymers (or plastics) are made up of smaller molecular units called monomers. This concept has led to the development of various types of plastics such as polyethylene (PE), polystyrene (PS), polypropylene (PP), and myriads of other polymers derived from the fossil hydrocarbons, but surprisingly none of the earlier developed plastics were biodegradable [17]. Of the majority of plastics, only 6–14% are recycled, which means nearly >80% remain in the atmosphere in landfills and in natural environment or water bodies via innumerable pathways (Fig. 1), causing a serious threat to the macrocosm [18]. Plastic waste can be found in oceans [19], air [20], sediments [21], soils [22], and surface water. Some of the major commonly used commodity plastics such as PE, polyvinyl chloride (PVC), PP, polyethylene terephthalate (PET) and PS are [23,24] generally considered resistant to hydrolytic and enzymatic breakdowns [25]. The chemical structures of some of the major plastics are shown in Fig. 2a. Macroplastics (>25 mm size), mesoplastics (5–25 mm), microplastics (MPs) (<5 mm), and nanoplastics (NPs) (<100 nm) [26] are the result of breakdown of large-size plastic debris. For instance, seawater plastics can undergo fragmentation into smaller debris (Fig. 3).
Fig. 1.
Plastics loading and transport pathways in the environment. The data are reproduced from [18] with permission from ACS.
Fig. 2.
(a): Chemical structures of some non-biodegradable polymers [25]; (b–c) primary and secondary sources of MPs causing pollution in the environment.
Fig. 3.
Fragmentation and degradation flow and size-based differentiation of plastics [36] (reproduced with permission from ACS).
The origins of MPs/NPs can be either primary or secondary, depending upon their sources. The plastic beads used as exfoliant in personal care items [27], including industrial abrasives and accidental spills [28] are the best examples of primary MPs. In the United States alone via wastewater treatment plants (WWTPs), an estimated effluent of 8 trillion pieces of MPs (including microfibres) enter the aquatic media [29], thereby significantly adding the pollution of MPs to the environment [30–33]. The secondary MPs are the unintended results of larger plastics that can degrade in the atmosphere due to natural weathering processes such as biodegradation, hydrolysis, mechanical abrasion, and UV photodegradation. Examples of the secondary MPs include discarded fishing nets, ropes, plastics bags, car tires, and agricultural plastics mulches [28,34,35]. The primary and secondary sources of MPs are mainly responsible for enhanced environmental pollution (see Fig. 2b and c).
Owing to the indiscriminate nature of feeding habits, filter feeders and planktonic suspensions may be most vulnerable to microplastics ingestion [37]. PE, PP, and PS are less denser than seawater, making them buoyant and accessible to planktonic organisms [38]. MPs are injected by marine organisms and are translocated beyond the gut [39,40]. Terrestrial research on the ingestion of MPs for soil species are emerging [41,42], and plastics reach the soil primarily via aerial deposition, contaminated irrigation water due to the presence of nitrates [43], while MPs residues are left in agriculture or horticulture products [44]. In agricultural areas of Europe, the first 10 cm of the soil has been polluted with 670 plastics fibers/kg of the soil, of which >1000 to 4000 particles of MPs per/kg of dry matter were detected [45,46]. MPs and NPs were also detected in foodstuffs such as table salt [47], honey [48], sugar, and milk [49]. For instance, average contents of MPs reported in honey are 0.166 fibers/g and 0.009 fragments/g, and for table salt, the contents of MPs range between 0.007 and 0.68 particles/g [50]. MPs have also been detected in aquatic animals such as crabs, seabirds, oysters, and Antarctic krill, lugworms [51–53]. Thus, studies to detect these plastics materials require more powerful diagnostic tools to identify, classify, and accurately quantify them from the environment. Therefore, there is an urgent need to develop novel technologies to remove or reduce micro and nanoplastic levels from natural sources such as environment and water for the benefit of both wellness of human for sustaining the green environment. This review attempts to compile the literature findings and to critically discuss the data on the analysis of emerging MPs and NPs to understand their harmful effects to the environment. Various methods used for the mitigation of MPs and NPs along with analytical techniques used for their characterizations are also covered.
2. Sampling techniques of MPs
2.1. Water sampling
Depending on the properties of MPs viz., density, shape, size, chemical adsorption, and biofouling and environmental factors such as water density, wind, tides, and waves, MPs can be distributed in the water column. Consequently, the recovered MPs’ quantity and consistency are highly reliant on the position and depth of sampling. For the sampling of large water quantities often in situ use of sieves, nets, or pumps can be used, whose mesh size can significantly affect their contents. For instance, in case of nylon net, 100 μm can be 0.1 MP L−1, which is almost 100-times greater than the manta net (333 μm) having a concentration of 0.00135 MP L−1 [54]. However, due to clogging with suspended organic and mineral products, plankton nets need to be deployed for a limited period, reducing the amount of sampled water. For the separation of fibers, a mesh of 80 μm can filter 250-times more fibers than the mesh of 330 μm [55]. Sample bottle and rotating drum samplers are often used to sample MPs in the water column at various depths [56].
Another method used for assessing the MPs’ concentration in the ocean is the continuous plankton recorder [57]. The merits and demerits of the water sample collection type are: (i) Neuston and Manta net, which are easy to use to produce a large number of MPs for further testing, but they suffer from the demerits of expensive equipment and boat; the method can also be time-consuming with a lower detection limit of 333 μm; (ii) plankton net, which is easy to handle requiring samples with medium volumes of water; the lowest limit of detection with this method can be 100 μm, and the limitations of this method are expensive, requirement of the boat, and the static sampling requires a flow of water; (iii) sieving, wherein the collection of samples can be easier with no specialized equipment, but the method is time-consuming; pumps, where the samples of a larger volume of water are required with the choice of mesh size, but it has the risk of potential contamination.
2.2. Sediments sampling
This technique allows the evaluation of MPs in benthic sediments in sea-floor, the estuaries, and the sea [58]. Grab samplers have been commonly used in large quantities to gather surface sediments, and sediments from the Swedish coastal region were gathered with the Eckman grab by Noren [59], but the historical deposition details could not be obtained from this device due to the mixing of layers and the sediment disturbance; however, this drawback was resolved using the sediment corers [56]. The precise estimation of MPs concentration in the sediments involves the concept of sampling depth, as the top 1–5 cm layers have higher concentrations than the top 10 cm, and the number of replicates since 11 samples were recommended to estimate MPs concentration at 90% confidence level per 100 m of the beach [60].
2.3. Biota sampling
Biological evaluation entails the analysis of MPs consumed by the organisms during feeding. The biota sampling techniques rely on the type of movement by the animals involved: sedentary, active, slow, or fast runners, but the bird sampling relies on retrieving dead species (carcasses) at marine or coastal nesting sites. A study on recovered carcasses based on evaluating their stomach contents in the North sea was done to track and provide the ecological quality [61].
2.4. Sample processing of MPs
For quantification and characterization, MPs are to be isolated from water and sediment samples. These samples may then be exposed to two separate steps: (i) a reduction step that enables the sample volume to be reduced, such as example by using the nets during collection followed by sieving, and (ii) separation is generally done via density separation or filtration.
2.5. Density separation and filtration
Depending on the nature of plastic material and the fabrication method, specific density of plastics particles can differ considerably. Density difference was used to distinguish the plastics from the sediments (2.7 g cm−3), generally by mixing the salt-saturated solution with sediments and collecting the supernatant containing MPs for the filtration purpose [62].
Density separation by utilizing water can recover certain types of plastics (PE, PP) from the soil samples [63] or fibers from the sediments owing to their form, shape, and wide surface [64]. NaCl has been the most preferred salt for use in density separation [65] since it can achieve slightly higher densities with a higher extraction efficacy for bulkier polymers (high-density polyethylene). Quinn et al., [64] observed that NaCl (1.2 g cm−3) and NaBr (1.4 g cm−3) could reduce the recovery rates (<90%), but with larger errors even though NaI (1.6 g cm−3) and ZnBr2 (1.7 g cm−3) are capable of differentiating the heavier polymers with excellent recovery rate up to 99%. Additionally, single washing of the sediment may be required for the separation using ZnBr2 and NaI, while NaCl demands more washing.
In elutriation, water is generally poured at the bottom of a column, enabling the buoyant MPs to be isolated from the settling of organic matters and sediments. In the column, MPs are gathered in a mesh and then separated by thick solutions [66]. The benefits of elutriation are that MPs can be isolated effectively from the large sediment volumes, and the process can be environmental-friendly [67]. While isolating the MPs from water samples and supernatants containing plastics, filtering or sieving can be the most effective method of density separation; the lower size of MPs detected can be calculated by the pores or mesh sizes. However, the use of sieves with different mesh sizes makes it possible to differentiate various MPs’ size categories. In order to distinguish plastics from other materials such as organic debris, shell pieces, seagrasses, glass, and paint coatings, etc., a careful visual sorting of the residues is necessary, which can be done directly by the naked eye examination of the sample or with the help of a dissecting microscope [68,69].
2.6. Sampling of NPs
For the sampling of NPs, Hilderbrandt et al., [70] used the continuous flow centrifugation to maintain NPs (~160 nm) from ultrapure water as well as the filtered and unfiltered waters from the German Elbe River. The retention ability of NPs in ultrapure water could be varied, depending on the pump rates from 92 ± 8% (1 L h−1) to 53% ± 5% (5 L h−1%) in the river water. Here, the approved plankton content was used that retained quantitatively up to 23.0 ± 2.2% of an effective dose of the spiked NPs in the first centrifuge rotor, while the remaining fraction of NPs was recovered in the second fraction rotor with an effluent of 53% ± 5% [24.4 ± 2.4% (uc)]. Thus, due to the demonstrated potential for the separation, continuous flow centrifugation was proved to be a quite promising NPs sampling and enrichment technique. Although some NPs separation techniques have been documented, adapting the methods developed for the engineered NPs are successful such as magnetic field flow fractionation (MFFF), gel fractionation, size-exclusion chromatography (SEC), and electrophoresis [71].
In MFFF, the magnetic field separates the NPs flowing through the channels, which can tangentially pull magnetic particles in the direction of flow [72]. Adjusting the MFFF to NPs separation may require a magnetization technique that may be more complex than with the MPs as magnetic nanoparticles can be too big to be effective to bind with NPs. For sample sizing, SEC can be useful where the sample can be passed through a porous bead column, and while passing through porous matrix of the beads, smaller particles can slow down, but larger particles can pass around the beads, thus smaller particles leave the last column [73]. In case of nanoparticles, both these techniques are used at concentrations far higher than the predicted environmentally relevant NP concentrations.
3. Identification and analysis methods of MPs and NPs
3.1. Fourier transform infrared spectroscopy (FTIR)
Using FTIR, carbon-based plastics can be identified and bond compositions of the plastics materials can be distinguished from other inorganic and organic moieties [74]. FTIR was first used for MPs identification in both water samples and sediments [57,75–77]. FTIR coupling with micro-FTIR allowed non-destructive and non-invasive fragments and microfibres in-situ and infrared absorption of various plastics have been reported [78–80]. Generally, reflectance spectra were used to identify the non-infrared transparent samples such as filters and focal plane array (FPA) detectors have been useful to obtain spatially resolved spectra on an array of n × n pixels, each referring to an individual IR spectrum. This method helps to distinguish small fragments and microfibres over larger areas in lesser time [81,82].
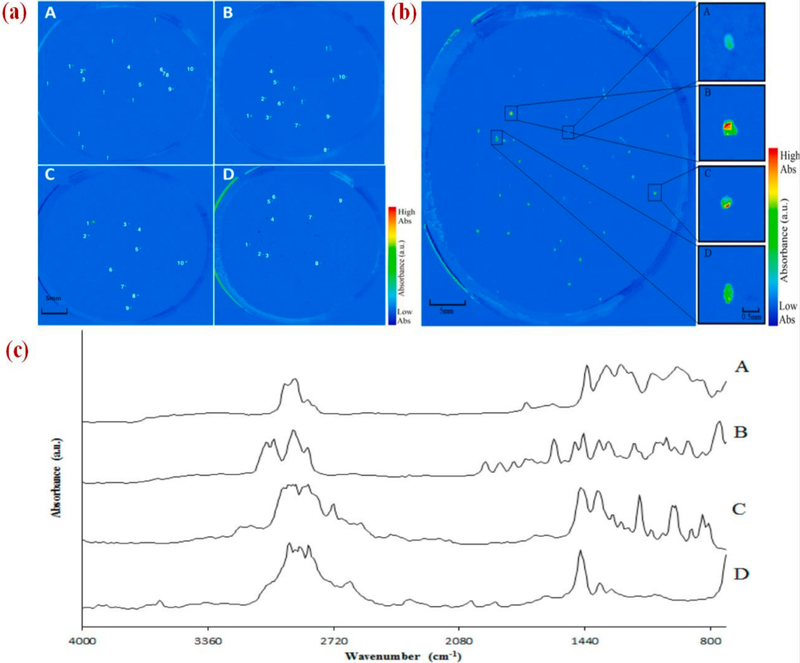
Focal plane array (FPA)-based reflectance micro-Fourier-transform (FT-IR) imaging and a pre-treatment stage using 30% hydrogen peroxide (H2O2) was used by Tagg et al., [81] to identify and image different types of microplastics (PE, PP, PVC, PS, and Nylon-6). The H2O2 pre-treatment was used to facilitate the filtration and to ameliorate micro-FT-IR imaging of the MPs in wastewater. Perkin Elmer spotlight micro-FT-IR equipped with Hg-Cd-telluride FPA detector was used (aperture size 25 μm × 25 μm). False-color images of the individual MPs on a 47 mm membrane filter are shown in Fig. 4a, and the filters were scanned to develop the images of plastic types in a single image as shown in Fig. 4b with FT-IR spectra (Fig. 4c) from the individual MPs fragments. Based on Fig. 4a and b, appropriate identification of microplastics (PVC, PP, PS, and PE) can be made by combining 30% H2O2 pre-treatment step with FPA micro-FT-IR imaging. Nearly 98% of this technique based on several correctly recognized MPs fragments are shown in Table 1.
Fig. 4.
(a): False-color images of membrane filters, each map displaying a polymer type, (A) PE; (B) PP; (C) PS; and (D) PVC. Correctly recognized spiked MPs are denoted 1–10 and additional MPs present in wastewater are denoted by “!”; (b) Combined false-color images of different MPs types; (c) FT-IR spectra of MPs fragments were (A) PVC; (B) PS; (C) PP; and (D) PE [81] (reproduced with permission from ACS).
Table 1.
Experimental data validation.
| MPs | Replicates | Fragments spiked | Fragments successfully recognized | Spiked fragments successfully recognized |
|---|---|---|---|---|
| Polyethylene | 1 | 10 | 12 | 10 |
| 2 | 10 | 17 | 10 | |
| 3 | 10 | 12 | 10 | |
| Polypropylene | 1 | 10 | 10 | 10 |
| 2 | 10 | 14 | 10 | |
| 3 | 10 | 9 | 9 | |
| Polyvinyl chloride | 1 | 10 | 10 | 10 |
| 2 | 10 | 9 | 9 | |
| 3 | 10 | 18 | 10 | |
| Polystyrene | 1 | 10 | 10 | 10 |
| 2 | 10 | 10 | 10 | |
| 3 | 10 | 10 | 10 | |
| Total | 120 | 131 | 118 | |
| Success rate (%) | 98 |
Cincinelli et al., [83] evaluated the presence of MPs in sub-surface water of the Ross sea using a non-invasive method to analyze MPs by FT-IR 2D imaging with an FPA detector. Cary 620e670 FTIR microscope, equipped with an FPA (Focal Plane Array) 128 × 128 detector (Agilent Technologies), was used to directly detect MPs fibers onto the dried filters. MPs ranged from 0.0032 to 1.18 particles per m3 of seawater with an average of 0.17 ± 0.34 particle m−3, indicating the concentrations below those observed in oceans around the world. The FTIR imaging of different types of MPs concluded the abundance of PE and PP in these samples.
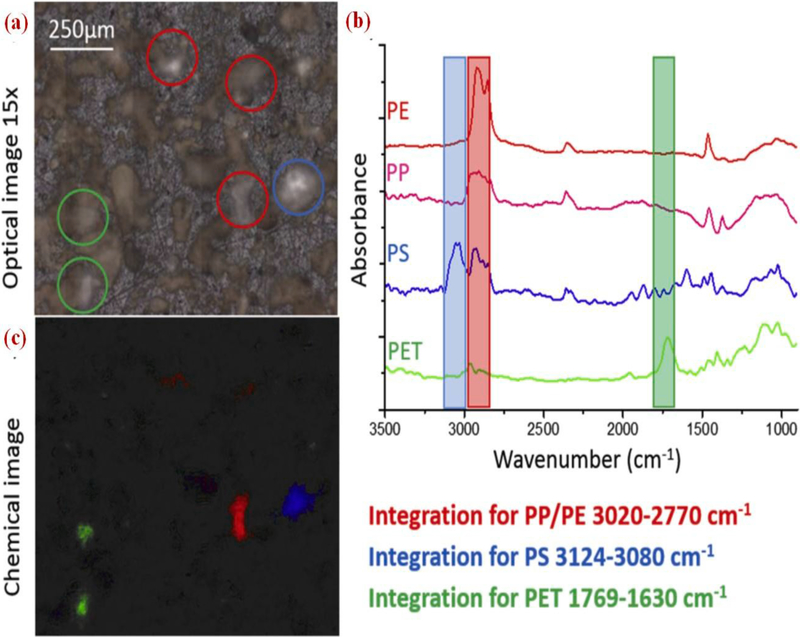
Elert et al., [84] used FTIR Vertex 70 spectrometer (Bruker) as a beam source, coupled with a Hyperion 3000 FTIR microscope to detect the MPs. Samples were distributed uniformly on a KBr crystal for each type of plastics chosen to differentiate the MPs from the soil (PET band between 1769 and 1630 cm−1, PP and PE 3020 and 2770 cm−1, and for PS 3124–3080 cm−1). The optical presentation on KBr crystal of reference material (RM) sample is shown in Fig. 5a, and bands are chosen specifically for the plastics as shown in Fig. 5b. For four plastics, simultaneous integration of three characteristic bands is displayed in Fig. 5c. The visual picture (Fig. 5a) demonstrates darker particles of sand and lighter particles of plastics placed on KBr can be detected easily, which helps to monitor the number and size of the particles in a given sample. The authors concluded that by using 1 mg of sample for FTIR imaging identification, particle size distribution of PE, PP, PS, and PET was done, but quantification could not be done by this technique.
Fig. 5.
(a): Optical image of RM sample; (b) Spectra of the particles marked with the circles marked; (c) chemical image showing integration wavelengths specified [84] (reproduced with permission from Elsevier).
Overall, FT-IR is a relatively reliable and quick method used to detect MPs; the results are summarized in Table 2.
Table 2.
Summary FT-IR results for the analysis of MPs.
| Location | Sample used | Abundance of MPs | Ref. |
|---|---|---|---|
| Yellow River, China | Surface water | 1760 ± 710 to 10,120 ± 4090 MPsm−3 | [85] |
| Mediterranean sea | Seawater; Fish | Seawater: 0.23 ± 0.20MPs m−3
Sardine: 0.20 ± 0.69 MPs per individual Anchovy: 0.11 ± 0.31 per individual |
[86] |
| Bizerte lagoon | Mollusc | From 703.95 ± 109.80 to 1482.82 ± 19.20 MPs kg−1 wet weight. | [87] |
| River Ticino, Italy | Eurasian otter | 2 MPs were identified out of 24 suspect particles. | [88] |
| Pacific sea | Sea turtle | 828 MPs ingested from 50 sea turtles. | [89] |
| Mondego estuary (Portugal) | Fish | 1.67 ± 0.27 MPs per fish. | [90] |
| Western Lake Superior | Surface water | Mean: 1200 mg km−2; Range: 91–3538 mg km−2 (mass per unit area). | [91] |
| Germany | Drinking water | Mean: 0.7 MPs m−3; Range: 0–7 MPs m−3. | [92] |
| Yellow sea, China | Sediments; Benthic organisms | Sediment: 560–4205 MPs kg−1 dry weight;Benthic organisms: 1.7–47 MPs kg−1 wet weight. |
[93] |
| Hong Kong | Benthic sediments | Mean: 189 +_50 MPs kg−1; Range: 169–221 MPs kg−1. | [94] |
| Germany | Farmland | 0.34 ± 0.36 MPs kg−1 dry weight. | [95] |
| Indian coast | Sediment | From 45 ± 12 to 220 ± 50 MPs kg−1 dry weight. | [96] |
| Norway | Sediment | 12,000–200,000 MPs kg−1 dry weight. | [97] |
3.2. Raman spectroscopy
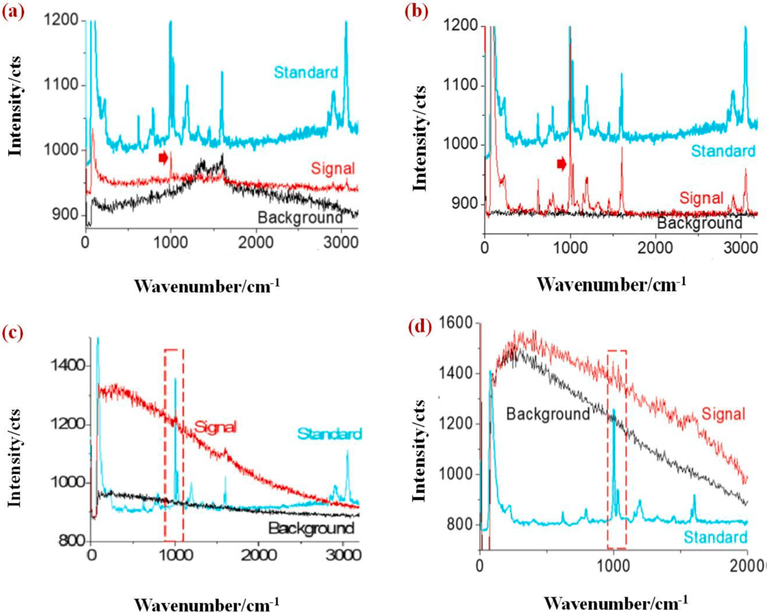
Raman imaging provides a direct target visualization, which is gaining much attention compared to FT-IR, since Raman offers a highly specific fingerprint sensor and higher spectral coverage [98]. Sobhani et al., [99] employed the Raman imaging to identify MPs and NPs down to 100 nm. For imaging polystyrene NPs, a Raman signal at 1000 cm−1 was chosen. Initially, NPs with a diameter of 600 nm were tested and identified from the Raman spectra as shown in Fig. 6a, where the arrowed peak was used for mapping. The background spectrum in Fig. 6a is originated from the amorphous carbon having two peaks at ~1350 cm−1 and ~1580 cm−1. Glass surface or gold-coated silicon water surface was used for sample preparation to get a clean background and flat surface for the distribution of NPs. Raman spectra shown in Fig. 6b shows a clean background confirming that NPs are made up of PS and NPs of diameter 300 nm that were studied on a gold slide surface (Fig. 6c). However, due to the smaller size (300 nm), Raman signal was not strong (Fig. 6b), and NPs with a diameter 100 nm were studied on a gold slide surface (Fig. 6d). Because of the smaller size of NPs (100 nm), Raman signal was weak, but a meaningful mapping image was generated.
Fig. 6.
(a–b): Raman spectra of NPs with a diameter of 600 nm; (c) spectra of NPs with 300 nm diameter prepared on gold slide surface; (d) Raman spectra of NPs with 100 nm of size [99] (reproduced with permission from Elsevier).
Zhang et al., [100] used Raman imaging and scanning electron microscopy (RISE) to study the NPs release from the recycled PVC powder to demonstrate that recycled PVC powder is made up of PVC and CaCO3. Raman spectra of PVC showed absorption peaks at 639 and 697 cm−1 that are assigned to C-Cl stretching vibrations and absorbance peaks at 1178, 1432, and 2917 cm−1 corresponding to C–H rocking, C–H bending, and C–H stretching vibration, respectively. These studies showed the direct identification of NPs released from the recycled plastics. Lv et al., [101] analyzed the MPs and NPs in pure water and seawater using surface-enhanced Raman spectroscopy (SERS) in which a silver colloid was used as an active substrate for qualitative analysis and NaCl was the aggregating agent for silver colloid. Raman signals of MPs and NPs showed good enhancement efficiency with an optimal enhancement factor of 4 × 104. In pure water, SERS signals of 100 nm and 500 nm polystyrene, absorption peak was at 1000 cm−1. In seawater, SERS signals showed a similar trend. Thus, SERS-based methods can detect 100 nm plastics down to 40 μg/mL concentration. SERS technique was also used to characterize organic aerosol particles (secondary) and fine atmospheric particles [102,103].
Xu et al., [104] detected and identified the MPs using SERS over klarite substrate, which is a dense grid with a shape of inverted pyramidal cavities made up of gold. Raman spectra of PS particles with sizes from 360 nm to 5 μm were collected, but the spectra of PS particles on silicon wafers were difficult to detect as the silicon Raman spectra dominated the signal. Only PS particles of size 5 μm showed a peak at 1003 cm−1, while smaller PS particles showed a peak at 521, 800–1000 cm−1, signifying the Raman signal of silicon only. Interestingly, Raman spectra of sizes 2 and 5 μm of PS particles were observed onto klarite substrate; two peaks were observed at 1003 and 1033 cm−1 due to C–C and C–H vibrations of the monosubstituted aromatic compounds were observed. The Raman spectra of PMMA (5 μm) onto silicon wafers was observed at 1453 cm−1, which was dominated by the spectra of the silicon substrate, while that for PMMA on the klarite surface was seen at 622 cm−1 due to C–C–O stretching, 817 cm−1 (C–O–C symmetric stretching), 1000 cm−1 (C–C stretching), 1452 cm−1 (C–H bending), 1723 cm−1 (C=O stretching), suggesting that SERS with klarite substrate helped to detect the NPs in the environment.
Table 3 summarizes literature reports of the detection of microplastics using Raman technique. Different researchers have collected MPs samples from various sources and locations where these were profusely present to conclude that maximum MPs were present in water samples instead of soil, biota or atmosphere. For spotting the MPs, Raman spectroscopy was found to be the best identifying method for the characterisation of MPs, which is crucial for the evaluation of potential environmental impacts.
Table 3.
A summary of the literature data obtained using Raman technique for the analysis of MPs.
| Location | Sample source | MPs abundance | Ref. |
|---|---|---|---|
| The wastewater treatment plant, Germany | Wastewater | 3000–5900 MPsm−3 | [105] |
| Three Gorges reservoir, China | Water; Sediment | Water: 1597–12,611 MPs m−3; Sediment: 25–300 MPs kg−1 wet weight |
[106] |
| Bavarianm, Germany | Bottled mineral water | From 2649 ± 2857 MPs L−1 to 6292 ± 10521 MPs L−1 | [107] |
| French Pyrenees | Atmosphere | Daily counts of 249 fragments, 73 films, and 44 fibers per square meter | [108] |
| Australian and Malaysian markets | Canned fish | 1–3 MPs per brand in 4 brands | [109] |
| North London lake, UK | Sediment | 539 MPs kg−1 dry weight | [110] |
| Qinghai Lake, China | Water; River; Sediment; Fish | Surface water: 0.05 × 105−7.58 × 105 MPs km−2 River: 0.03 × 105–0.31 × 105 MPs km−2 Sediment: 50–1292 MPs m−2 Fish: 2–15 MPs per individual |
[110] [111] |
| Snake and lower Columbia Rivers | Surface water | From 0 to 5.405 MPs L−1 and 0–0.014 MPs L−1 | [112] |
| Tibet Plateau | Surface water Sediment | Water: 483–967MPs m−3
Sediment: 50–195 MPskg−1 |
[113] |
| Southeast Spain | Sea surface | 0.10 ± 0.09 MPsm−2 | [114] |
| Xiangjiang River, China | Sediment | From 270.17 ± 48.23 MPs kg−1 to 866.59 ± 37.96 MPskg−1 | [115] |
| Danjiangkou Reservoir, China | Water; Sediment | Water: 467–15,017 MPsm−3; Sediment: 15–40 MPs kg−1 net weight |
[116] |
3.3. Surface identification methods for MPs and NPs
3.3.1. Time of flight secondary ion mass spectroscopy (ToF-SIMS)
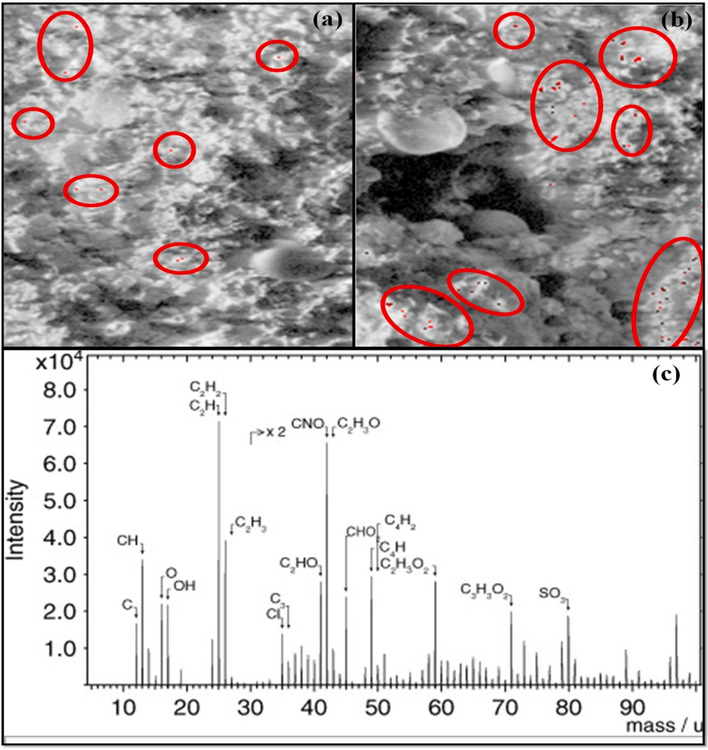
ToF-SIMS is a versatile method for the surface analysis of materials since its outstanding mass resolution and high spatial resolution can provide a wealth of knowledge for a large array of materials [117]. Jungnickel et al., [118] used ToF-SIMS to detect polyethylene MPs in the environment. The Ottawa sand was collected and used for the exposure to sea surf simulation and identification of the characteristics ions was done. When the analysis was done in a positive mode, the prevalent fragment ions for PE obtained were [C3H3(CH2)n]+, [CnH2n − 1]+, and [CnH2n + 1]+. The fragment ion, m/z 113, was considered appropriate for the PE identification as it has a discriminating power for all the matrices studied. Na, K, and Ca were the absorbed ionic species onto the sample surface, and as displayed in Fig. 7a, after 14 days exposure to sea surf simulation, degradation of PE pellet surface was observed, signifying the conversion of larger particles into smaller ones, but the detected size of small particles was increased by 50% when the exposure time was increased to 1 month (Fig. 7b).
Fig. 7.
ToF-SIMS images of total ions shown in greyscale and ion m/z 113 shown in red from the polyethylene (PE) pellets (a) when PE pellets were exposed for 14 days to sea surf simulation; (b) exposed for 1 month to sea surf simulation [118] (reproduced with permission from Elsevier); (c) ToF-SIMS spectra of the oxidized surface of SW pellet [119]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Du et al., [120] studied twelve different soil samples collected from China to detect MPs characterized by ToF-SIMS combined with a high-resolution mode. The four samples of MPs identified were PP, PVC, PET, poly(amide 6) (PA6). The highest proportion observed was for PET and PA6, accounting for 30.2% and particle sizes varying from 0 to 35 μm. Additionally, Kern et al., [121] provided the database of ToF-SIMS reference spectra of plastics to identify the primary ion species such as bismuth-cluster in both positive and negative polarity. Du et al., also used [122] ToF-SIMS to characterize the MPs found in farmlands and their distribution characteristics were analyzed. The authors found fragment ions that were used for the detection: m/z = 71.086 for polypropylene, m/z = 30.036 (CH4N+) for poly(amide 6), m/z = 149.024 for polyethylene terephthalate, and m/z = 83.978 (C4HCl+).
Biesinger et al., [119] used ToF-SIMS for understanding the breakdown of plastics in freshwater (FW) beaches and seawater (SW) beach plastics. The ToF-SIMS imaging of PE samples emphasized more light on the species that were contributing to the oxidation process. The mass spectra associated with the spectra of positive ion of PE for both FW and SW pellets are CnH2n+1 and CnH2n−1 series and C, CH, CH2, C2, C2H, C3, C3H, C3H2, C3H3, C4, C4H, and C4H3 for the negative ion spectra, while the oxidized PE fragments for both FW and SW consists of O, OH, C2HO, C2H2O, C2H3O, C2H5O, C2H2O2, C2H3O2, C3H3O2 for the negative ions spectra and CH3O for the positive ion spectra, suggesting that oxidized fragments prominently appeared in the negative ion spectra (Fig. 7c).
3.3.2. Matrix-assisted laser desorption/ionization-time of flight mass spectroscopy (MALDI TOF MS)
Matrix-assisted laser desorption/ionization-time of flight mass spectroscopy is an effective technique for ionizing and detecting the intact large molecular weight molecules [123]; the technique uses three components viz., ionization, separation, and detection [124]. The samples can be vaporized and ionized onto a sample plate made up of stainless steel, and mass spectrometry isolated the analytes under various voltages to detect them based on their mass-to-charge ratios. MALDI TOF MS has also attracted much interest in identifying and quantifying the emerging contaminants of the environment with high benefits of sensitivity, throughput analysis, and simple operation [125,126].
Wu et al., [127] used MALDI TOF MS for the detection of micro (nano)plastics (MNPs) in the environment, wherein a quantitative correlation was established between the normalized signal and concentration of the plastic with a correlation coefficients of > 0.96 and 0.98 for low and high molecular weight plastics, respectively. Two types of environmental samples of MNPs, including aviation cup particles and aged MNPs from the river sediments were prepared and when analyzed using MALDI – TOF MS, polystyrene related MNPs (in both aviation cup and sediment) were found to consist of C8H8 and C16H16O oligomers. In contrast, PET-related MNPs (only found in the sediment) were detected with the repeat units of C10H8O4 and C12H12O4. The contents of polystyrene and PET MNPs were found to be 8.56 ± 0.04 and 28.71 ± 0.20 mg·kg−1, respectively in the collected sediment.
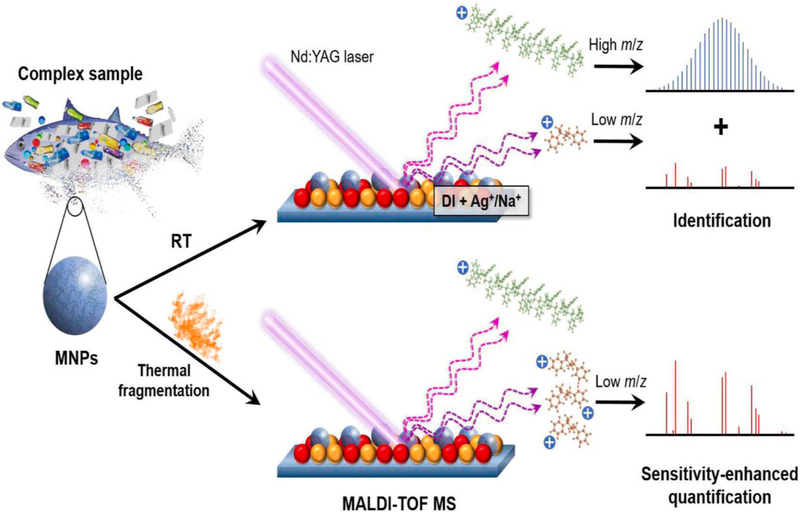
Weidner et al., [128] and Dimzon et al., [129] also used MALDI TOF MS for the detection of different types of plastics, while Kirstein et al., [130] studied the identification of MPs attached Vibrio on plastics. Lin et al., [131] used PS particles as model micro/nano plastic (MNPs) to investigate their thermal fragmentation and identification (Fig. 8). The fingerprint peaks of PS MNPs in low mass (m/z 90, 104, 128, 130, and 312–318) and high mass regions (same peaks with Δm/z 104 in the range of m/z (350–5000) were obtained and quantified with m/z 315.3.
Fig. 8.
Scheme showing the procedure and mechanism for defining and quantifying thermally enhanced process of PS MNPs by MALDI TOF MS at room temperature [131] (reproduced with permission from Elsevier).
3.3.3. Inductively coupled plasma mass spectroscopy (ICP-MS)
Inductively coupled plasma mass spectrometry has been a useful method for efficient trace metal analysis [132–134]; when operated in a single event mode, it can be used to characterize MPs and NPs. This technique is known as single-particle (SP) ICP-MS, which gives information on particle mass concentration, particle number density, chemical composition, and size distribution [134,135]. Lamana et al., [136] used this method to detect and quantify the NPs for conjugated NPs with functionalized gold (Au) nanoparticles. Here, the adsorbed Au particles generated in the SP ICP-MS signal count the individual NPs particles, thereby giving an accurate quantification. The quantification limit of NPs was calculated to be 8.4 × 105 NPTs L−1, and the established calibration graph was linear up to 3.5 × 108 NPTs L−1. This method was applied to analyze the NPs up to 1 μm in drinking, tap, and river waters. Hydrodynamic chromatography (HDC) coupled with ICP MS was also used to characterize MPs from various environmental sites, which proved that HDC ICP-MS is a promising technique for detecting the MPs in complex media. For instance, MPs of PS assessed by this method showed the sizes of 50, 100, 200, 300, and 500 nm where density of PS was 1.04 g/cm3 [137]. In general, ICP MS technique was useful for detecting a wide array of MPs [138–141] and NPs [70,136,142] from the polluted environment.
4. Effect of MPs and NPs on biomass (aquatic organism/soil microorganism/biomolecules)
Characteristics such as high endurance, longer stability, and small size make MPs and NPs deleterious for aquatic organisms, animals, and birds [34]. MPs and NPs have been identified in most aquatic creatures such as freshwater crustaceans, marine copepod, mongonont rotifer, amphipod, beach hopper, barnacles, and many more. The possible dangers caused by the ingestion of MPs and NPs, their type, particle size and concentration, are summarized in Table 4. The existence of PS MPs (0.1%) in the soil environment showed an increase in retention time for antibiotics and antibiotic-resistant genes (ARGs) [143]. Lei et al., exposed Caenorhabditis elegans (soil invertebrate) to PS particles (0.1, 0.5, 1.0, 2.0, and 5.0 μm) for three days to observe that 1.0 μm had the lowest survival rate, shortest life span, and the highest decrease in body length, while the highest toxicity of 1.0 μm PS particle was attributed to the nematodes taking moderately-sized plastics more readily [144]. It was also reported that other MPs such as polyurethane (PU), polyamide (PA) and polyethersulfone (PES) have the positive effects on aquatic systems.
Table 4.
Effects of MPs and NPs on aquatic organisms.
| Organism | Plastics type | Particle size | Concentration | Effects | Ref. |
|---|---|---|---|---|---|
| Freshwater crustacean (Daphnia magna) | PET | 62–1,400 μm | 12.5–100 mg/L (48 h exposure and subsequent 24 h recovery) | Increased mortality of daphnia and failure of daphnia to recover from MP exposure | [147] |
| PE | 1, 100 μm | 12.5–400 mg/L (96 h exposure) | Ingestion of MP led to dose- and time-dependent immobilization | [148] | |
| Marine copepod (Paracyclopina nana) | PS | 0.05, 0.5 and 6 μm | 0–20 μg/mL (24 h exposure followed by 24 h recovery | Developmental delays and decrease in fecundity, delayed moulting, increased ROS levels, phosphorylation of MAPK, and antioxidant enzymatic activities of GPx, GR, GST, and SOD | [149] |
| Monogonont rotifer (Brachionus koreanus) | PS | 0.05, 0.5 and 6 μm | 0.1, 1, 10 and 20 μg/mL (12- day exposure) 10 μg/mL (48 h exposure | Reduced growth rate, fecundity and lifespan, and longer reproduction time. Antioxidant-related enzymes and MAPK signaling pathways were significantly activated | [150] |
| Freshwater amphipod (Hyalella azteca) | PP | 0–90 MP/mL (10-day exposure) 0–22.5 MP/mL (42- day exposure) | Reduced growth and reproduction | [151] | |
| Barnacles (Megabalanus azoricus) | PVC | 1.5 μm | 0, 0.003, 0.03, 0.3 and 3% (6- week exposure | Reduced cirral activity and oxygen Consumption | [152] |
| Three-spined stickleback (Gasterosteus aculeatus) | PS | Мm 9.9 μm |
1.81–1010 particles/mL (7-day exposure, 14-day recovery) 1.81–107 particles/mL (7-day exposure, 14-day recovery) | Prolonged food digestion, effects on length, weight, and condition index K were observed | [153] |
| 0.5 μm | 1.4 _ 1014 particles/mL (7-day exposure, 14-day recovery) | ||||
| Lugworms (Arenicola marina | Polyacetic acid (PLA) | 1.4–707 μm | 0.02, 0.2 and 2% of sediment WW (31-day exposure) | Metabolic rates increased, while microalgal biomass decreased | [154] |
| PE | 2.5–316 μm | ||||
| PVC | 8.7–478 μm | ||||
| Zebrafish larvae (Danio rerio) | PS | 45 μm 50 nm |
1 mg/L (120 h exposure) | MP upregulated zfrho visual gene expression, whereas NP inhibited larval locomotion and acetylcholinesterase activity to significantly reduce larvae body length and upregulated gfap, α1-tubulin, zfrho and zfblue gene expression significantly | [155] |
The level of exposure is important to study the effect of MPs on soil organisms (Oligochaeta). In this regard, Zhu et al., [145] investigated the effect of concentration on PS nanoplastics on soil oligochaete, which showed that 10% have a significant negative effect compared to 0.025% and 0.5%. Similar results were also reported by Huerta Lwanga et al., [41], where they showed that 28–60% of PE MPs had the inhibition effect on earthworm, while 7% PE MPs did not affect the survival and growth of the earthworm. Hollóczki and Gehrke [146] studied the adverse effects of NPs on biomolecules to show that NPs can interact with proteins to modify the functionally of secondary structure of biomolecules and denature them. Thus, adverse effects of MPs and NPs can be possible on every sphere of the macrocosm as shown in Fig. 9.
Fig. 9.
MPs and NPs showing detrimental effects on different components of the environment and finally their effects on food chain.
5. Efficient and cost-effective treatment processes for the removal of MPs and NPs
5.1. MPs removal via membrane technology
Membrane technology has been powerful for the mitigation of plastic litter from wastewater, primarily because membrane-based operations can replace most of the traditional energy-intensive technologies due to their low energy requirement, versatility, ease of operation, good stability, simple control, and scale-up possibility. To our surprise not much has been studied in the literature using this approach.
5.1.1. Wastewater
Wastewater is a significant contributor of MPs to rivers, seas, and oceans because they cannot be extracted by the current standard screening methods due to small size of the primary MPs. Since wastewater is treated and regulated in most countries worldwide, this could be easily avoided. Ultrafiltration (UF) is a well-known and most suitable alternative for water treatment since its low energy requirement and high efficiency of separation renders it an economically viable process to achieve high-quality drinking water [156]. UF is a low-pressure driven process (1–10 bar) to reject particulates, macromolecules, and suspended solid particles using asymmetric UF membranes (pore sizes of 1–100 nm). Among the other methods, UF has been widely explored for wastewater treatment such as secondary and tertiary filtrations [156].
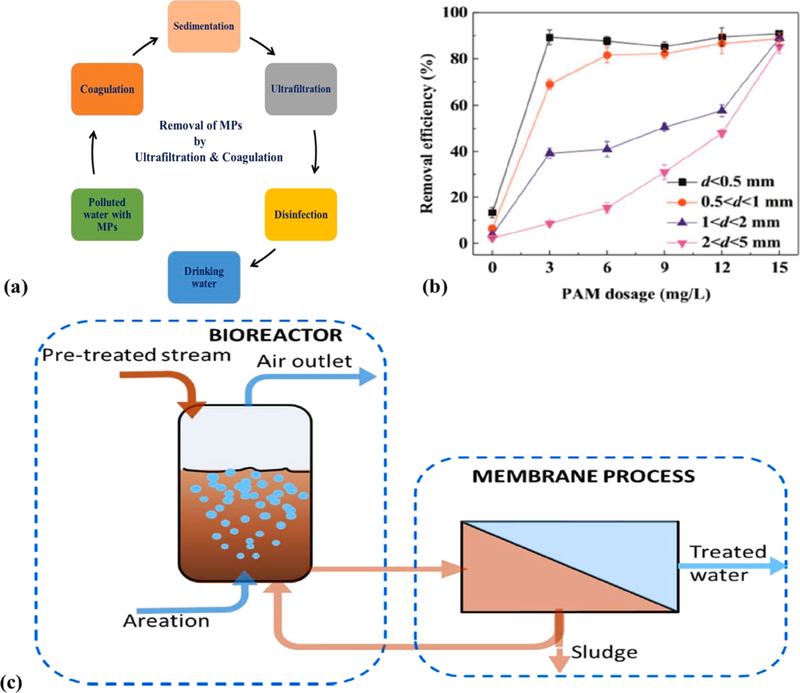
Coupling coagulation with UF can be an excellent choice to improve effluent quality of the discharged pollutants (Fig. 10a). Ma et al., [157] have investigated the removal of most abundant plastics pollutants such as polyethylene using Fe-based coagulant via UF, where the efficacy of PE particles was low (<15%), showing the ineffectiveness of single coagulation. However, by the addition of anionic polyacrylamide (PAM) [158] it was possible to boost the coagulation capacity and removal efficacy of PE particles (d < 0.5 mm) from 13 to 90.9% (see Fig. 10b). This was possible due to the dense floc formation and high adsorption capability of Fe-based flocks (positively charged) under neutral conditions. The authors also studied the effect of Al and Fe-based salts and PE particles as they can be easily floated/suspended in water, the prime constituents of MPs. The results indicated that Al-based salts improved the PE removal efficiency even though removal efficiency (40%) was observed at a high dosage (15 mM) of Al-based salt. Though UF completely rejected the PE particles, but membrane fouling occurred due to the coagulation of Al-based salts. In any case, the combination of coagulation and UF was recommended to be a better approach for the removal of MPs than the singly used technique for applications in wastewater treatment containing MPs and NPs [159].
Fig. 10.
(a): Scheme of the process of removal of MPs by UF and coagulation; (b) removal efficacy of PE using anionic PAM; (c) schematic representation of the membrane bioreactor (MBR) process [173].
Membrane bioreactors (MBRs) are other types of advanced waste treatment techniques containing biological catalyst-supported catalysis (enzymes or bacteria) systems that are coupled to membrane-based separation (microfiltration or ultrafiltration) [160]. MBRs coupled with artificial intelligence act as a sustainable tool in treating wastewater [161]. MBRs were also used in the treatment of industrial biowastes [162] and effluents [163]. In many types of wastewater treatment, MBR was successfully applied to remove the emerging pollutants such as antibiotics, pesticides, pharmaceuticals, and plastics including personal care products [164]. While treating the MPs by MBR, the filtration step’s complexity can be reduced first by the biodegradation of organic matter, thus allowing the MPs to be separated efficiently from wastewater. In an MBR system, first MPs are treated in a bioreactor in the presence of bacteria or enzymes, where organic matter biodegradation occurs, and then separation can be achieved to remove the plastics’ materials (see schematics of MBR process in Fig. 10c).
Talvitie et al., [33] proposed many approaches to increase the rejection of MPs up to 99.9% using MBRs, which could replace conventional coagulation-precipitation methods after the activated sludge aeration (secondary treatment). The downside of this study was that MPs with sizes > 20 μm were considered. Lares et al., [164] studied the efficiency of a municipal wastewater treatment plant located in Mikkeli, Finland to remove MPs from wastewater by collecting samples once in every two weeks for three months of sampling campaigns. WWTP was employed based on the conventional activated sludge (CAS) process and a pilot-scale MBR to find that MBR had a better removal efficiency (99.4%) compared to the overall CAS-based process (98.3%). In their study, PET was divulged to be the most abundant in collected samples, constituting 79% of the entire MPs load [165]. Lv. et al., [166] studied the removal of MPs in a full scale WWTP (Eastern China) using two parallel wastewater treatment systems that are oxidation ditch (OD) and MBR. The influent MPs were removed by 99.5% in the MBR system, while 97% was removed by the OD system on the basis of plastic mass, which removed 82.1% and 53.6%, respectively, suggesting that MBR can be significantly superior to OD in removing MPs due to membrane-based filtration.
Dynamic membrane technology (DMT) has recently emerged as an appealing technology for urban wastewater treatment [167], surface water treatment [168], industrial wastewater treatment, and the treatment of sludge. The technology is focused on the creation of a cake layer dynamic membrane (DM), which functions as a secondary membrane/barrier created by supporting membrane filters and foulants in wastewater, where membrane can be cleaned by surface brushing [168] and air scouring [169]. The DM formation depends on different parameters relating to the supporting membrane, the matter deposited, operating pressure, and the velocity of cross-flow [167]. Li. et al., [170] employed dynamic membrane technology to remove MPs to demonstrate that around 90% of the retained MPs of particle size < 90 μm were removed.
Zhang et al., [171] studied the removal of MPs in different processes of the leachate treatment system to find removal efficiencies of MPs up to 16.67%, 50%, 20%, and 75% after the adjustment tank, MBR (membrane bioreactor), two stage AO (anionic/oxic), and UF (ultrafiltration), respectively, but NF (nanofiltration) and RO (reverse osmosis) showed no effect on MPs removal. Pizzichetti et al., [172] evaluated the performance of membranes for MPs removal using a simple, low-cost system that could be implemented in a domestic environment. For the removal of polyamide and polystyrene MPs (20–300 μm), the output of polycarbonate, cellulose acetate, and polytetrafluoroethylene membranes with the same nominal pore size of 5 μm was assessed to achieve high mass removal efficiencies (>94% for both types of MPs). Variable performances were observed due to differences in the interaction between MPs and membrane, shape irregularity, and membrane hardness. It was found that for the mass removal efficiency of MPs, CA (cellulose acetate) was found to be the ideal membrane for the implementation of a domestic household system.
5.2. Removal of NPs
Wang et al., [172] examined the efficient removal of polystyrene NPs from water using a low-pressure driven electro-spun membrane with a tuned surface charge. Three modified membranes viz., M, M0, and M + with enhanced mechanical strength and hydrophilicity, respectively displayed negative, neutral, and positive charges at pH 7.2 were synthesized by alternatively assembling three layers of polyethylenimine (PEI) and poly(acrylic acid) (PAA) on the hydrolyzed electro-spun PAN poly(acrylonitrile) membrane. The PS (polystyrene) NPs (50 nm) were much smaller than the membrane pores to be efficiently removed by the M + membrane (89%) from wastewater [174]. Busse et al., [175] successfully fractionated varying the pore size (from several μm to hundreds of nm) NPs using a 2.5 μm cellulose filter; the MPs and NPs were separated by immersing the plastic teabags at 95 °C; here, polytetrafluoroethylene (PTFE) filter with the pore size of 0.2 μm was used to separate NPs.
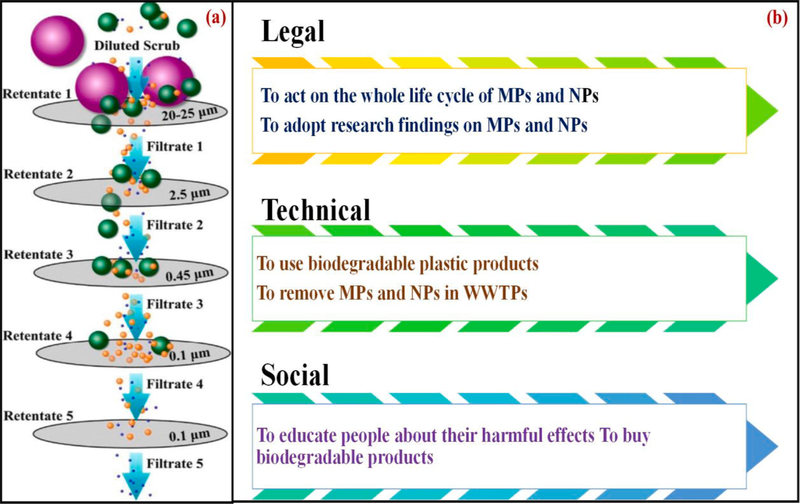
A sequential filtration method using diverse pore sized membranes was also used by Hernandez et al., [27] in a five-step filtration process (Fig. 11a) using 25 μm, 2.5 μm, 0.45 μm, and two 0.1 μm pore size membranes for the separation of different of sized PE NPs. In another study [174], pre-concentration, isolation, and purification of NPs were achieved by UF in which hydrostatic pressure was applied to a nano-porous membrane to separate the plastics particles. The authors also used a crossflow UF system in drinking water samples for the removal of NPs, wherein a recovery of 50 nm size PS spheres was removed [176], but efficiency of this approach was abysmal (12%).
Fig. 11.
(a): Scheme for the sequential filtration of NPs of PE in industrial facial scrub [27]; (b) legal, technical, and social measures for the mitigation and risk assessment of MPs and NPs contamination.
Field flow fractionation (FFF) is another effective technique for the separation of environmental NPs, which can be adapted for the size discrimination of NPs. An optimized asymmetrical flow FFF (AF4) method was developed by Gigault et al., [176] to sort out the NPs of PS in colloidal size ranges from 1 to 800 nm. The method showed the efficiency to enable high-resolution sub-fraction discrimination viz., 10–100 nm, 100–200 nm, 200–450 nm, and 450–800 nm in diameter demonstrating its powerful separation ability. NPs in the environmental water and aquatic food were also isolated by this method [142]. The advantage of AF4 is that it could isolate and characterize the nanoparticles by coupling them to online detectors simultaneously [177].
The removal performance of MPs is dependent on chemical or biological treatment technologies such as membrane bioreactors, biologically active fillers, disc filtration, sand filtration, activated carbon filtration, coagulation/flocculation, air flotation, reverse osmosis, and advanced oxidation [178–182]. For instance, coagulation can remove MPs from 47.1 to 81.6% [178], while membrane bioreactors that have the pore size of 0.4 μM can remove up to 99.9% [179]. Sand filtration and air flotation showed the removal efficiencies up to 97% and 95%, respectively [179]. Advanced oxidation processes such as Fenton agent, H2O2-UV system, ozone-UV systems, and ultrasound-UV systems have also the potential to remove MPs due to the formation of radicals that can cause chain breaking of the MPs, thereby losing physico-chemical characteristics of the MPs such as molecular weight, density, size, etc. Hydrophobic iron nanoparticles were introduced into the dissolved air flotation process for capturing MPs by the magnetic force. This process has been used to recover > 1 mm size of different MPs such as PE, PP, PVC PU, and PET from seawater [181].
6. Challenges in assessing and mitigating the plastics
Many challenges remain in the risk evaluation of MPs and NPs due to significant information gaps between their quantity in the environment, environmental activities, and exposure pathways. Researchers have studied the risk assessments and provided a comprehensive data-basis [181,182] to understand the NPs pollution strategies with regard to (i) creation of innovative remediation technologies, (ii) policymaking, and (iii) public awareness (see Fig. 11b) as well as to suggest the most successful approach to prevent the NPs from entering into the marine environments
Of the many technologies employed in wastewater treatment plants, engineering aspects are intended to eradicate NPs. Pre-treatment strategies for removing MPs in drinking water, including density separation and coagulation aas well as membrane-based separations have proven successful. However, their ability to remove NPs requires further examination [181]. The risk mitigation strategies of NPs in terrestrial environments have been investigated with a minimal study. The available literature indicates that adding biochar [182] to the waste might decrease the NPs migration in porous media, thereby mitigating the risks through retention/stabilization [183]. Since plastic goods are an integral part of everyday life cycle, raising public awareness of MPs and NPs may be a viable and beneficial option for managing their potential risks.
7. Conclusions and future perspectives
The presence of MPs and NPs highly pollutes the Earth’s environment. The present review summarizes various sampling methods and studies used in the mitigation and further characterization of MPs and NPs. The review also provides an overview of the hazardous effects caused by the plastics as emerging pollutants. WWTP and wastewater purification industries are currently not effective technologies to distinguish the MPs from the waste sources. To remove MPs and NPs from wastewater, advanced membrane processes need to be fully utilized to explore further environmentally benign methods such as ultrafiltration, dynamic membrane technology, reverse osmosis, membrane disc filters, and membrane bioreactors. MBR can also be a promising approach among these processes with 99% removal of MPs. Techniques including field flow fractionation have also been discussed for the removal of NPs.
In addition to the strategies to reduce plastic usage and to identify alternative material sources, a better understanding of MPs and NPs elimination technologies is crucial. Future studies on the mitigation of MPs and NPs need to prioritize better and more innovative sampling methods. A more elaborated and detailed sequence of methods for chemical-physical characterization and identification of MPs and NPs is need of the day. Finally, risk assessment and awareness among the public and design of operation and production-based processes on the usage of biodegradable plastics materials is necessary to mitigate these toxic pollutants to provide the green environment.
Acknowledgment
Ms. Aayushi Kundu is thankful to TIET-Virginia Tech Center of Excellence in Emerging Materials, India for fellowship.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer
This document has been reviewed in accordance with U.S. Environmental Protection Agency policy and approved for publication. Any mention of trade names, manufacturers or products does not imply an endorsement by the United States Government or the U.S. Environmental Protection Agency. EPA and its employees do not endorse any commercial products, services, or enterprises.
References
- [1].Moharir RV, Kumar S, Challenges associated with plastic waste disposal and allied microbial routes for its effective degradation: a comprehensive review, J. Clean. Prod. 208 (2019) 65–76, 10.1016/j.jclepro.2018.10.059. [DOI] [Google Scholar]
- [2].Sharma S, Kundu A, Basu S, Shetti NP, Aminabhavi TM, Sustainable environmental management and related biofuel technologies, J. Environ. Manage. 273 (2020) 111096. [DOI] [PubMed] [Google Scholar]
- [3].Srivastava RK, Shetti NP, Reddy KR, Aminabhavi TM, Sustainable energy from waste organic matters via efficient microbial processes, Sci. Total Environ. 137927 (2020). [DOI] [PubMed] [Google Scholar]
- [4].Mehta A, Mishra A, Basu S, Shetti NP, Reddy KR, Saleh TA, Aminabhavi TM, Band gap tuning and surface modification of carbon dots for sustainable environmental remediation and photocatalytic hydrogen production–a review, J. Environ. Manage. 250 (2019) 109486. [DOI] [PubMed] [Google Scholar]
- [5].Sharma S, Basu S, Shetti NP, Aminabhavi TM, Waste-to-energy nexus for circular economy and environmental protection: recent trends in hydrogen energy, Sci. Total Environ. 713 (2020) 136633. [DOI] [PubMed] [Google Scholar]
- [6].Mishra A, Basu S, Shetti NP, Reddy KR, Aminabhavi TM, Photocatalysis of graphene and carbon nitride-based functional carbon quantum dots, in: Nanoscale Mater. Water Purif., Elsevier, 2019, pp. 759–781. [Google Scholar]
- [7].Davarazar M, Mostafaie A, Jahanianfard D, Davarazar P, Ghiasi SAB, Gorchich M, Nemati B, Kamali M, Aminabhavi TM, Treatment technologies for pharmaceutical effluents-a scientometric study, J. Environ. Manage. 254 (2020) 109800, 10.1016/j.jenvman.2019.109800. [DOI] [PubMed] [Google Scholar]
- [8].Reddy NL, Rao VN, Vijayakumar M, Santhosh R, Anandan S, Karthik M, Shankar MV, Reddy KR, Shetti NP, Nadagouda MN, A review on frontiers in plasmonic nano-photocatalysts for hydrogen production, Int. J. Hydrogen Energy 44 (2019) 10453–10472. [Google Scholar]
- [9].Reddy NR, Bhargav U, Kumari MM, Cheralathan KK, Shankar MV, Reddy KR, Saleh TA, Aminabhavi TM, Highly efficient solar light-driven photocatalytic hydrogen production over Cu/FCNTs-titania quantum dots-based heterostructures, J. Environ. Manage. 254 (2020) 109747, 10.1016/j.jenvman.2019.109747. [DOI] [PubMed] [Google Scholar]
- [10].Mishra A, Shetti NP, Basu S, Reddy KR, Aminabhavi TM, Recent developments in ionic liquid-based electrolytes for energy storage supercapacitors and rechargeable batteries, in: Green Sustain. Process Chem. Environ. Eng. Sci., Elsevier, 2020, pp. 199–221. [Google Scholar]
- [11].Mishra A, Shetti NP, Basu S, Raghava Reddy K, Aminabhavi TM, Carbon cloth-based hybrid materials as flexible electrochemical supercapacitors, ChemElectroChem 6 (2019) 5771–5786. [Google Scholar]
- [12].Mishra A, Mehta A, Basu S, Malode SJ, Shetti NP, Shukla SS, Nadagouda MN, Aminabhavi TM, Electrode materials for lithium-ion batteries, Mater. Sci. Energy Technol. 1 (2018) 182–187. [Google Scholar]
- [13].Srivastava RK, Shetti NP, Reddy KR, Aminabhavi TM, Biofuels, biodiesel and biohydrogen production using bioprocesses. A review, Environ. Chem. Lett. 18 (2020) 1049–1072, 10.1007/s10311-020-00999-7. [DOI] [Google Scholar]
- [14].Navakoteswara Rao V, Lakshmana Reddy N, Mamatha Kumari M, Ravi P, Sathish M, Kuruvilla KM, Preethi V, Reddy KR, Shetti NP, Aminabhavi TM, Shankar MV, Photocatalytic recovery of H2 from H2S containing wastewater: Surface and interface control of photo-excitons in Cu2S@TiO2 core-shell nanostructures, Appl. Catal. B Environ. 254 (2019) 174–185. 10.1016/j.apcatb.2019.04.090. [DOI] [Google Scholar]
- [15].Reddy KR, Reddy CV, Nadagouda MN, Shetti NP, Jaesool S, Aminabhavi TM, Polymeric graphitic carbon nitride (g-C 3 N 4)-based semiconducting nanostructured materials: Synthesis methods, properties and photocatalytic applications, J. Environ. Manage. 238 (2019) 25–40, 10.1016/j.jenvman.2019.02.075. [DOI] [PubMed] [Google Scholar]
- [16].Wright SL, Kelly FJ, Plastic and human health: a micro issue? Environ. Sci. Technol. 51 (2017) 6634–6647. [DOI] [PubMed] [Google Scholar]
- [17].Geyer R, Jambeck JR, Law KL, Production, use, and fate of all plastics ever made, Sci. Adv. 3 (2017) e1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alimi OS, Farner Budarz J, Hernandez LM, Tufenkji N, Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport, Environ. Sci. Technol. 52 (2018) 1704–1724. [DOI] [PubMed] [Google Scholar]
- [19].da Costa JP, Santos PSM, Duarte AC, Rocha-Santos T, (Nano) plastics in the environment–sources, fates and effects, Sci. Total Environ. 566 (2016) 15–26. [DOI] [PubMed] [Google Scholar]
- [20].Panko JM, Chu J, Kreider ML, Unice KM, Measurement of airborne concentrations of tire and road wear particles in urban and rural areas of France, Japan, and the United States, Atmos. Environ. 72 (2013) 192–199. [Google Scholar]
- [21].Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M, Microplastics in the marine environment: a review of the methods used for identification and quantification, Environ. Sci. Technol. 46 (2012) 3060–3075. [DOI] [PubMed] [Google Scholar]
- [22].Nizzetto L, Bussi G, Futter MN, Butterfield D, Whitehead PG, A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments, Environ. Sci. Process. Impacts. 18 (2016) 1050–1059. [DOI] [PubMed] [Google Scholar]
- [23].Rochman CM, Hoh E, Hentschel BT, Kaye S, Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris, Environ. Sci. Technol. 47 (2013) 1646–1654. [DOI] [PubMed] [Google Scholar]
- [24].da Costa JP, Paço A, Santos PSM, Duarte AC, Rocha-Santos T, Microplastics in soils: assessment, analytics and risks, Environ. Chem. 16 (2019) 18–30. [Google Scholar]
- [25].Ng EL, Huerta Lwanga E, Eldridge SM, Johnston P, Hu HW, Geissen V, Chen D, An overview of microplastic and nanoplastic pollution in agroecosystems, Sci. Total Environ. 627 (2018) 1377–1388, 10.1016/j.scitotenv.2018.01.341. [DOI] [PubMed] [Google Scholar]
- [26].Jahnke A, Arp HPH, Escher BI, Gewert B, Gorokhova E, Kühnel D, Ogonowski M, Potthoff A, Rummel C, Schmitt-Jansen M, Reducing uncertainty and confronting ignorance about the possible impacts of weathering plastic in the marine environment, Environ. Sci. Technol. Lett. 4 (2017) 85–90. [Google Scholar]
- [27].Hernandez LM, Yousefi N, Tufenkji N, Are there nanoplastics in your personal care products? Environ. Sci. Technol. Lett. 4 (2017) 280–285, 10.1021/acs.estlett.7b00187. [DOI] [Google Scholar]
- [28].McDevitt JP, Criddle CS, Morse M, Hale RC, Bott CB, Rochman CM, Addressing the issue of microplastics in the wake of the microbead-free waters act - a new standard can facilitate improved policy, Environ. Sci. Technol. 51 (2017) 6611–6617, 10.1021/acs.est.6b05812. [DOI] [PubMed] [Google Scholar]
- [29].Rochman CM, Kross SM, Armstrong JB, Bogan MT, Darling ES, Green SJ, Smyth AR, Veríssimo D, Scientific evidence supports a ban on microbeads, Environ. Sci. Technol. 49 (2015) 10759–10761, 10.1021/acs.est.5b03909. [DOI] [PubMed] [Google Scholar]
- [30].Pivokonsky M, Cermakova L, Novotna K, Peer P, Cajthaml T, Janda V, Occurrence of microplastics in raw and treated drinking water, Sci. Total Environ. 643 (2018) 1644–1651, 10.1016/j.scitotenv.2018.08.102. [DOI] [PubMed] [Google Scholar]
- [31].Murphy F, Ewins C, Carbonnier F, Quinn B, Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment, Environ. Sci. Technol. 50 (2016) 5800–5808. [DOI] [PubMed] [Google Scholar]
- [32].Wang Z, Lin T, Chen W, Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP), Sci. Total Environ. 700 (2020) 134520, 10.1016/j.scitotenv.2019.134520. [DOI] [PubMed] [Google Scholar]
- [33].Talvitie J, Mikola A, Koistinen A, Setälä O, Solutions to microplastic pollution – removal of microplastics from wastewater effluent with advanced wastewater treatment technologies, Water Res. 123 (2017) 401–407, 10.1016/j.watres.2017.07.005. [DOI] [PubMed] [Google Scholar]
- [34].Sharma S, Basu S, Shetti NP, Nadagouda MN, Aminabhavi TM, Microplastics in the environment: occurrence, perils, and eradication, Chem. Eng. J. (2020), 127317, 10.1016/j.cej.2020.127317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hernandez E, Nowack B, Mitrano DM, Polyester textiles as a source of microplastics from households: a mechanistic study to understand microfiber release during washing, Environ. Sci. Technol. 51 (2017) 7036–7046. [DOI] [PubMed] [Google Scholar]
- [36].Gillibert R, Balakrishnan G, Deshoules Q, Tardivel M, Magazzù A, Donato MG, Maragò OM, Lamy M De La Chapelle, F. Colas, F. Lagarde, P. G. Gucciardi, Raman tweezers for small microplastics and nanoplastics identification in seawater, Environ. Sci. Technol. 53 (2019) 9003–9013, 10.1021/acs.est.9b03105. [DOI] [PubMed] [Google Scholar]
- [37].Setälä O, Norkko J, Lehtiniemi M, Feeding type affects microplastic ingestion in a coastal invertebrate community, Mar. Pollut. Bull. 102 (2016) 95–101. [DOI] [PubMed] [Google Scholar]
- [38].Andrady AL, Microplastics in the marine environment, Mar. Pollut. Bull. 62 (2011) 1596–1605. [DOI] [PubMed] [Google Scholar]
- [39].Rochman CM, Browne MA, Underwood AJ, Van Franeker JA, Thompson RC, Amaral-Zettler LA, The ecological impacts of marine debris: unraveling the demonstrated evidence from what is perceived, Ecology 97 (2016) 302–312. [DOI] [PubMed] [Google Scholar]
- [40].Galloway TS, Cole M, Lewis C, Interactions of microplastic debris throughout the marine ecosystem, Nat. Ecol. Evol. 1 (2017) 1–8. [DOI] [PubMed] [Google Scholar]
- [41].Huerta Lwanga E, Gertsen H, Gooren H, Peters P, Salánki T, Van Der Ploeg M, Besseling E, Koelmans AA, Geissen V, Microplastics in the terrestrial ecosystem: implications for Lumbricus terrestris (Oligochaeta, Lumbricidae), Environ. Sci. Technol. 50 (2016) 2685–2691. [DOI] [PubMed] [Google Scholar]
- [42].Rodriguez-Seijo A, Lourenço J, Rocha-Santos TAP, Da Costa J, Duarte AC, Vala H, Pereira R, Histopathological and molecular effects of microplastics in Eisenia andrei Bouché, Environ. Pollut. 220 (2017) 495–503. [DOI] [PubMed] [Google Scholar]
- [43].Kamali M, Davarazar M, Aminabhavi TM, Single precursor sonochemical synthesis of mesoporous hexagonal-shape zero-valent copper for effective nitrate reduction, Chem. Eng. J. 384 (2020) 123359, 10.1016/j.cej.2019.123359. [DOI] [Google Scholar]
- [44].Arthur C, Baker J, Bamford H, International research workshop on the occurrence, effects, and fate of microplastic marine debris, Conf. Proceedings.Sept (2008) 9–11. [Google Scholar]
- [45].Zubris KAV, Richards BK, Synthetic fibers as an indicator of land application of sludge, Environ. Pollut. 138 (2005) 201–211. [DOI] [PubMed] [Google Scholar]
- [46].Barnes DKA, Galgani F, Thompson RC, Barlaz M, Accumulation and fragmentation of plastic debris in global environments, Philos. Trans. R. Soc. B Biol. Sci. 364 (2009) 1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kim J-S, Lee H-J, Kim S-K, Kim H-J, Global pattern of microplastics (MPs) in commercial food-grade salts: sea salt as an indicator of seawater MP pollution, Environ. Sci. Technol. 52 (2018) 12819–12828. [DOI] [PubMed] [Google Scholar]
- [48].Liebezeit G, Liebezeit E, Non-pollen particulates in honey and sugar, Food Addit. Contam. Part A. 30 (2013) 2136–2140. [DOI] [PubMed] [Google Scholar]
- [49].Coppock RL, Cole M, Lindeque PK, Queirós AM, Galloway TS, A small-scale, portable method for extracting microplastics from marine sediments, Environ. Pollut. 230 (2017) 829–837. [DOI] [PubMed] [Google Scholar]
- [50].Presence of microplastics and nanoplastics in food, with particular focus on seafood, EFSA J. 14 (2016) e04501. 10.2903/j.efsa.2016.4501. [DOI] [Google Scholar]
- [51].Foley CJ, Feiner ZS, Malinich TD, Höök TO, A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates, Sci. Total Environ. 631 (2018) 550–559. [DOI] [PubMed] [Google Scholar]
- [52].Jinhui S, Sudong X, Yan N, Xia P, Jiahao Q, Yongjian X, Effects of microplastics and attached heavy metals on growth, immunity, and heavy metal accumulation in the yellow seahorse, Hippocampus kuda Bleeker, Mar. Pollut. Bull. 149 (2019) 110510. [DOI] [PubMed] [Google Scholar]
- [53].Van Cauwenberghe L, Claessens M, Vandegehuchte MB, Janssen CR, Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats, Environ. Pollut. 199 (2015) 10–17. [DOI] [PubMed] [Google Scholar]
- [54].Vermaire JC, Pomeroy C, Herczegh SM, Haggart O, Murphy M, Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries, Facets 2 (2017) 301–314. [Google Scholar]
- [55].Dris R, Gasperi J, Rocher V, Tassin B, Synthetic and non-synthetic anthropogenic fibers in a river under the impact of Paris Megacity: sampling methodological aspects and flux estimations, Sci. Total Environ. 618 (2018) 157–164, 10.1016/j.scitotenv.2017.11.009. [DOI] [PubMed] [Google Scholar]
- [56].Thevenon F, Carroll C, Sousa J, Plastic debris in the ocean: the characterization of marine plastics and their environmental impacts, situation analysis report, Plast. Debris Ocean Charact. Mar. Plast. Their Environ. Impacts, Situat. Anal. Rep. 52 (2015), 10.2305/iucn.ch.2014.03.en. [DOI] [Google Scholar]
- [57].Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AWG, McGonigle D, Russell AE, Lost at sea: where is all the plastic? Science (Washington) 304 (2004) 838. [DOI] [PubMed] [Google Scholar]
- [58].Claessens M, De Meester S, Van Landuyt L, De Clerck K, Janssen CR, Occurrence and distribution of microplastics in marine sediments along the Belgian coast, Mar. Pollut. Bull. 62 (2011) 2199–2204. [DOI] [PubMed] [Google Scholar]
- [59].Sweden KIMO, Small plastic particles in Coastal Swedish waters, N-Research (2007) 11. [Google Scholar]
- [60].Besley A, Vijver MG, Behrens P, Bosker T, A standardized method for sampling and extraction methods for quantifying microplastics in beach sand, Mar. Pollut. Bull. 114 (2017) 77–83, 10.1016/j.marpolbul.2016.08.055. [DOI] [PubMed] [Google Scholar]
- [61].GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection, Sources, fate and effects of microplastics in the marine environment: a global assessment”, Reports Stud. GESAMP. 90 (2015) 96. issn: 1020–4873%5Cnhttp://ec.europa.eu/environment/marine/good-environmental-status/descriptor-10/pdf/GESAMP_microplasticsfullstudy.pdf. [Google Scholar]
- [62].Rocha-Santos T, Duarte AC, A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment, TrAC - Trends Anal. Chem. 65 (2015) 47–53, 10.1016/j.trac.2014.10.011. [DOI] [Google Scholar]
- [63].Zhang S, Yang X, Gertsen H, Peters P, Salánki T, Geissen V, A simple method for the extraction and identification of light density microplastics from soil, Sci. Total Environ. 616–617 (2018) 1056–1065, 10.1016/j.scitotenv.2017.10.213. [DOI] [PubMed] [Google Scholar]
- [64].Quinn B, Murphy F, Ewins C, Validation of density separation for the rapid recovery of microplastics from sediment, Anal. Methods. 9 (2017) 1491–1498, 10.1039/c6ay02542k. [DOI] [Google Scholar]
- [65].Nuelle MT, Dekiff JH, Remy D, Fries E, A new analytical approach for monitoring microplastics in marine sediments, Environ. Pollut. 184 (2014) 161–169, 10.1016/j.envpol.2013.07.027. [DOI] [PubMed] [Google Scholar]
- [66].Kedzierski M, Le Tilly V, César G, Sire O, Bruzaud S, Efficient microplastics extraction from sand. A cost effective methodology based on sodium iodide recycling, Mar. Pollut. Bull. 115 (2017) 120–129, 10.1016/j.marpolbul.2016.12.002. [DOI] [PubMed] [Google Scholar]
- [67].Kedzierski M, Le Tilly V, Bourseau P, Bellegou H, César G, Sire O, Bruzaud S, Microplastics elutriation from sandy sediments: a granulometric approach, Mar. Pollut. Bull. 107 (2016) 315–323, 10.1016/j.marpolbul.2016.03.041. [DOI] [PubMed] [Google Scholar]
- [68].Doyle MJ, Watson W, Bowlin NM, Sheavly SB, Plastic particles in coastal pelagic ecosystems of the Northeast Pacific ocean, Mar. Environ. Res. 71 (2011) 41–52, 10.1016/j.marenvres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- [69].Morét-Ferguson S, Law KL, Proskurowski G, Murphy EK, Peacock EE, Reddy CM, The size, mass, and composition of plastic debris in the western North Atlantic Ocean, Mar. Pollut. Bull. 60 (2010) 1873–1878. [DOI] [PubMed] [Google Scholar]
- [70].Hildebrandt L, Mitrano DM, Zimmermann T, Pröfrock D, A nanoplastic sampling and enrichment approach by continuous flow centrifugation, Front. Environ. Sci. 8 (2020), 10.3389/fenvs.2020.00089. [DOI] [Google Scholar]
- [71].Robertson JD, Rizzello L, Avila-Olias M, Gaitzsch J, Contini C, MagoÅ MS, Renshaw SA, Battaglia G, Purification of nanoparticles by size and shape, Sci. Rep. 6 (2016) 1–9, 10.1038/srep27494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Latham AH, Freitas RS, Schiffer P, Williams ME, Capillary magnetic field flow fractionation and analysis of magnetic nanoparticles, Anal. Chem. 77 (2005) 5055–5062, 10.1021/ac050611f. [DOI] [PubMed] [Google Scholar]
- [73].Flavel BS, Kappes MM, Krupke R, Hennrich F, Separation of single-walled carbon nanotubes by 1-dodecanol-mediated size-exclusion chromatography, ACS Nano 7 (2013) 3557–3564. [DOI] [PubMed] [Google Scholar]
- [74].Löder MGJ, Gerdts G, Methodology used for the detection and identification of microplastics—a critical appraisal, in: Mar. Anthropog. Litter, Springer, 2015, pp. 201–227. [Google Scholar]
- [75].Frias J, Otero V, Sobral P, Evidence of microplastics in samples of zooplankton from Portuguese coastal waters, Mar. Environ. Res. 95 (2014) 89–95. [DOI] [PubMed] [Google Scholar]
- [76].Reddy MS, Basha S, Adimurthy S, Ramachandraiah G, Description of the small plastics fragments in marine sediments along the Alang-Sosiya ship-breaking yard, India, Estuar. Coast. Shelf Sci. 68 (2006) 656–660. [Google Scholar]
- [77].Vianello A, Boldrin A, Guerriero P, Moschino V, Rella R, Sturaro A, Da Ros L, Microplastic particles in sediments of Lagoon of Venice, Italy: first observations on occurrence, spatial patterns and identification, Estuar. Coast. Shelf Sci. 130 (2013) 54–61. [Google Scholar]
- [78].Song YK, Hong SH, Jang M, Kang J-H, Kwon OY, Han GM, Shim WJ, Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer, Environ. Sci. Technol. 48 (2014) 9014–9021. [DOI] [PubMed] [Google Scholar]
- [79].Mansur H, Oréfice R, Pereira M, Lobato Z, Vasconcelos W, Machado L, FTIR and UV-vis study of chemically engineered biomaterial surfaces for protein immobilization, Spectroscopy. 16 (2002) 351–360. [DOI] [PubMed] [Google Scholar]
- [80].Mecozzi M, Pietroletti M, Monakhova YB, FTIR spectroscopy supported by statistical techniques for the structural characterization of plastic debris in the marine environment: application to monitoring studies, Mar. Pollut. Bull. 106 (2016) 155–161. [DOI] [PubMed] [Google Scholar]
- [81].Tagg AS, Sapp M, Harrison JP, Ojeda JJ, Identification and quantification of microplastics in wastewater using focal plane array-based reflectance micro-FT-IR imaging, Anal. Chem. 87 (2015) 6032–6040, 10.1021/acs.analchem.5b00495. [DOI] [PubMed] [Google Scholar]
- [82].Bhargava R, Wang S-Q, Koenig JL, FTIR microspectroscopy of polymeric systems, in: Liq. Chromatogr. Microspectrosc. Assist. Synth., Springer, 2003, pp. 137–191. [Google Scholar]
- [83].Cincinelli A, Scopetani C, Chelazzi D, Lombardini E, Martellini T, Katsoyiannis A, Fossi MC, Corsolini S, Microplastic in the surface waters of the Ross Sea (Antarctica): occurrence, distribution and characterization by FTIR, Chemosphere 175 (2017) 391–400. [DOI] [PubMed] [Google Scholar]
- [84].Elert AM, Becker R, Duemichen E, Eisentraut P, Falkenhagen J, Sturm H, Braun U, Comparison of different methods for MP detection: what can we learn from them, and why asking the right question before measurements matters? Environ. Pollut. 231 (2017) 1256–1264. [DOI] [PubMed] [Google Scholar]
- [85].Wang Z, Qin Y, Li W, Yang W, Meng Q, Yang J, Microplastic contamination in freshwater: first observation in Lake Ulansuhai, Yellow River Basin, China, Environ. Chem. Lett. 17 (2019) 1821–1830. [Google Scholar]
- [86].Lefebvre C, Saraux C, Heitz O, Nowaczyk A, Bonnet D, Microplastics FTIR characterisation and distribution in the water column and digestive tracts of small pelagic fish in the Gulf of Lions, Mar. Pollut. Bull. 142 (2019) 510–519. [DOI] [PubMed] [Google Scholar]
- [87].Abidli S, Lahbib Y, El Menif NT, Microplastics in commercial molluscs from the lagoon of Bizerte (Northern Tunisia), Mar. Pollut. Bull. 142 (2019) 243–252. [DOI] [PubMed] [Google Scholar]
- [88].Smiroldo G, Balestrieri A, Pini E, Tremolada P, Anthropogenically altered trophic webs: alien catfish and microplastics in the diet of Eurasian otters, Mammal Res. 64 (2019) 165–174. [Google Scholar]
- [89].Jung MR, Horgen FD, Orski SV, Rodriguez V, Beers KL, Balazs GH, Jones TT, Work TM, Brignac KC, Royer S-J, Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms, Mar. Pollut. Bull. 127 (2018) 704–716. [DOI] [PubMed] [Google Scholar]
- [90].Bessa F, Barría P, Neto JM, Frias JPGL, Otero V, Sobral P, Marques JC, Occurrence of microplastics in commercial fish from a natural estuarine environment, Mar. Pollut. Bull. 128 (2018) 575–584. [DOI] [PubMed] [Google Scholar]
- [91].Hendrickson E, Minor EC, Schreiner K, Microplastic abundance and composition in western Lake Superior as determined via microscopy, Pyr-GC/MS, and FTIR, Environ. Sci. Technol. 52 (2018) 1787–1796. [DOI] [PubMed] [Google Scholar]
- [92].Mintenig SM, Löder MGJ, Primpke S, Gerdts G, Low numbers of microplastics detected in drinking water from ground water sources, Sci. Total Environ. 648 (2019) 631–635. [DOI] [PubMed] [Google Scholar]
- [93].Wang J, Wang M, Ru S, Liu X, High levels of microplastic pollution in the sediments and benthic organisms of the South Yellow Sea, China, Sci. Total Environ. 651 (2019) 1661–1669. [DOI] [PubMed] [Google Scholar]
- [94].Cheang CC, Ma Y, Fok L, Occurrence and composition of microplastics in the seabed sediments of the coral communities in proximity of a metropolitan area, Int. J. Environ. Res. Public Health. 15 (2018) 2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Piehl S, Leibner A, Löder MGJ, Dris R, Bogner C, Laforsch C, Identification and quantification of macro-and microplastics on an agricultural farmland, Sci. Rep. 8 (2018) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Tiwari M, Rathod TD, Ajmal PY, Bhangare RC, Sahu SK, Distribution and characterization of microplastics in beach sand from three different Indian coastal environments, Mar. Pollut. Bull. 140 (2019) 262–273. [DOI] [PubMed] [Google Scholar]
- [97].Haave M, Lorenz C, Primpke S, Gerdts G, Different stories told by small and large microplastics in sediment-first report of microplastic concentrations in an urban recipient in Norway, Mar. Pollut. Bull. 141 (2019) 501–513. [DOI] [PubMed] [Google Scholar]
- [98].Ivleva NP, Wiesheu AC, Niessner R, Microplastic in aquatic ecosystems, Angew. Chemie Int. Ed. 56 (2017) 1720–1739. [DOI] [PubMed] [Google Scholar]
- [99].Sobhani Z, Zhang X, Gibson C, Naidu R, Megharaj M, Fang C, Identification and visualisation of microplastics/nanoplastics by Raman imaging (i): Down to 100 nm, Water Res. 174 (2020) 115658, 10.1016/j.watres.2020.115658. [DOI] [PubMed] [Google Scholar]
- [100].Zhang W, Dong Z, Zhu L, Hou Y, Qiu Y, Direct observation of the release of nanoplastics from commercially recycled plastics with correlative Raman imaging and scanning electron microscopy, ACS Nano 14 (2020) 7920–7926. [DOI] [PubMed] [Google Scholar]
- [101].Lv L, He L, Jiang S, Chen J, Zhou C, Qu J, Lu Y, Hong P, Sun S, Li C, In situ surface-enhanced Raman spectroscopy for detecting microplastics and nanoplastics in aquatic environments, Sci. Total Environ. 728 (2020) 138449, 10.1016/j.scitotenv.2020.138449. [DOI] [PubMed] [Google Scholar]
- [102].Fu Y, Kuppe C, Valev VK, Fu H, Zhang L, Chen J, Surface-enhanced Raman spectroscopy: a facile and rapid method for the chemical component study of individual atmospheric aerosol, Environ. Sci. Technol. 51 (2017) 6260–6267. [DOI] [PubMed] [Google Scholar]
- [103].Slade JH, Ault AP, Bui AT, Ditto JC, Lei Z, Bondy AL, Olson NE, Cook RD, Desrochers SJ, Harvey RM, Bouncier particles at night: biogenic secondary organic aerosol chemistry and sulfate drive diel variations in the aerosol phase in a mixed forest, Environ. Sci. Technol. 53 (2019) 4977–4987. [DOI] [PubMed] [Google Scholar]
- [104].Xu G, Cheng H, Jones R, Feng Y, Gong K, Li K, Fang X, Tahir MA, Valev VK, Zhang L, Surface-enhanced Raman spectroscopy facilitates the detection of microplastics <1 μm in the environment, Environ. Sci. Technol. (2020) acs. est.0c02317. 10.1021/acs.est.0c02317. [DOI] [PubMed] [Google Scholar]
- [105].Wolff S, Kerpen J, Prediger J, Barkmann L, Müller L, Determination of the microplastics emission in the effluent of a municipal waste water treatment plant using Raman microspectroscopy, Water Res. X. 2 (2019) 100014, 10.1016/j.wroa.2018.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Di M, Wang J, Microplastics in surface waters and sediments of the Three Gorges Reservoir, China, Sci. Total Environ. 616–617 (2018) 1620–1627, 10.1016/j.scitotenv.2017.10.150. [DOI] [PubMed] [Google Scholar]
- [107].Oßmann BE, Sarau G, Holtmannspötter H, Pischetsrieder M, Christiansen SH, Dicke W, Small-sized microplastics and pigmented particles in bottled mineral water, Water Res. 141 (2018) 307–316. [DOI] [PubMed] [Google Scholar]
- [108].Allen S, Allen D, Phoenix VR, Le Roux G, Jiménez PD, Simonneau A, Binet S, Galop D, Atmospheric transport and deposition of microplastics in a remote mountain catchment, Nat. Geosci. 12 (2019) 339–344. [Google Scholar]
- [109].Karami A, Golieskardi A, Choo CK, Larat V, Karbalaei S, Salamatinia B, Microplastic and mesoplastic contamination in canned sardines and sprats, Sci. Total Environ. 612 (2018) 1380–1386. [DOI] [PubMed] [Google Scholar]
- [110].Turner S, Horton AA, Rose NL, Hall C, A temporal sediment record of microplastics in an urban lake, London, UK, J. Paleolimnol. 61 (2019) 449–462. [Google Scholar]
- [111].Xiong X, Zhang K, Chen X, Shi H, Luo Z, Wu C, Sources and distribution of microplastics in China’s largest inland lake–Qinghai Lake, Environ. Pollut. 235 (2018) 899–906. [DOI] [PubMed] [Google Scholar]
- [112].Kapp KJ, Yeatman E, Microplastic hotspots in the Snake and Lower Columbia rivers: a journey from the Greater Yellowstone Ecosystem to the Pacific Ocean, Environ. Pollut. 241 (2018) 1082–1090, 10.1016/j.envpol.2018.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Jiang C, Yin L, Li Z, Wen X, Luo X, Hu S, Yang H, Long Y, Deng B, Huang L, Microplastic pollution in the rivers of the Tibet Plateau, Environ. Pollut. 249 (2019) 91–98. [DOI] [PubMed] [Google Scholar]
- [114].de Haan WP, Sanchez-Vidal A, Canals M, Party NSS, Floating microplastics and aggregate formation in the Western Mediterranean Sea, Mar. Pollut. Bull. 140 (2019) 523–535. [DOI] [PubMed] [Google Scholar]
- [115].Wen X, Du C, Xu P, Zeng G, Huang D, Yin L, Yin Q, Hu L, Wan J, Zhang J, Microplastic pollution in surface sediments of urban water areas in Changsha, China: abundance, composition, surface textures, Mar. Pollut. Bull. 136 (2018) 414–423. [DOI] [PubMed] [Google Scholar]
- [116].Di M, Liu X, Wang W, Wang J, Manuscript prepared for submission to environmental toxicology and pharmacology pollution in drinking water source areas: microplastics in the Danjiangkou Reservoir, China, Environ. Toxicol. Pharmacol. 65 (2019) 82–89. [DOI] [PubMed] [Google Scholar]
- [117].Sodhi RNS, Time-of-flight secondary ion mass spectrometry (TOF-SIMS):—versatility in chemical and imaging surface analysis, Analyst 129 (2004) 483–487. [DOI] [PubMed] [Google Scholar]
- [118].Jungnickel H, Pund R, Tentschert J, Reichardt P, Laux P, Harbach H, Luch A, Time-of-flight secondary ion mass spectrometry (ToF-SIMS)-based analysis and imaging of polyethylene microplastics formation during sea surf simulation, Sci. Total Environ. 563–564 (2016) 261–266, 10.1016/j.scitotenv.2016.04.025. [DOI] [PubMed] [Google Scholar]
- [119].Biesinger MC, Corcoran PL, Walzak MJ, Developing ToF-SIMS methods for investigating the degradation of plastic debris on beaches, Surf. Interface Anal. 43 (2011) 443–445. [Google Scholar]
- [120].Du C, Liang H, Li Z, Gong J, Pollution characteristics of microplastics in soils in southeastern suburbs of baoding city, China, Int. J. Environ. Res. Public Health 17 (2020) 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kern S, Kern C, Rohnke M, Mass spectra database of polymers for bismuth-cluster ToF-SIMS, Surf. Sci. Spectra 26 (2019) 25003. [Google Scholar]
- [122].Du C, Wu J, Gong J, Liang H, Li Z, ToF-SIMS characterization of microplastics in soils, Surf. Interface Anal. 52 (2020) 293–300. [Google Scholar]
- [123].Badía JD, Strömberg E, Ribes-Greus A, Karlsson S, A statistical design of experiments for optimizing the MALDI-TOF-MS sample preparation of polymers. An application in the assessment of the thermo-mechanical degradation mechanisms of poly (ethylene terephthalate), Anal. Chim. Acta 692 (2011) 85–95. [DOI] [PubMed] [Google Scholar]
- [124].Welker M, Fastner J, Erhard M, von Döhren H, Applications of MALDI-TOF MS analysis in cyanotoxin research, Environ. Toxicol. An Int. J. 17 (2002) 367–374. [DOI] [PubMed] [Google Scholar]
- [125].Payne ME, Grayson SM, Characterization of synthetic polymers via matrix assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry, JoVE (J. Vis. Exp.) (2018) e57174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Yang C, Lee HK, Zhang Y, Jiang L-L, Chen Z-F, Chung ACK, Cai Z, In situ detection and imaging of PFOS in mouse kidney by matrix-assisted laser desorption/ionization imaging mass spectrometry, Anal. Chem. 91 (2019) 8783–8788. [DOI] [PubMed] [Google Scholar]
- [127].Wu P, Tang Y, Cao G, Li J, Wang S, Chang X, Dang M, Jin H, Zheng C, Cai Z, Determination of environmental micro(nano)plastics by matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry, Anal. Chem. 92 (2020) 14346–14356, 10.1021/acs.analchem.0c01928. [DOI] [PubMed] [Google Scholar]
- [128].Weidner SM, Trimpin S, Mass spectrometry of synthetic polymers, Anal. Chem. 82 (2010) 4811–4829. [DOI] [PubMed] [Google Scholar]
- [129].Dimzon IKD, Knepper TP, MALDI–TOF MS for characterization of synthetic polymers in aqueous environment, in: Compr. Anal. Chem., Elsevier, 2012, pp. 307–338. [Google Scholar]
- [130].Kirstein IV, Kirmizi S, Wichels A, Garin-Fernandez A, Erler R, Löder M, Gerdts G, Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles, Mar. Environ. Res. 120 (2016) 1–8. [DOI] [PubMed] [Google Scholar]
- [131].Lin Y, Huang X, Liu Q, Lin Z, Jiang G, Thermal fragmentation enhanced identification and quantification of polystyrene micro/nanoplastics in complex media, Talanta 208 (2020) 120478, 10.1016/j.talanta.2019.120478. [DOI] [PubMed] [Google Scholar]
- [132].Mozhayeva D, Engelhard C, A critical review of single particle inductively coupled plasma mass spectrometry-a step towards an ideal method for nanomaterial characterization, J. Anal. At. Spectrom. 35 (2020) 1740–1783, 10.1039/c9ja00206e. [DOI] [Google Scholar]
- [133].Montaño MD, Olesik JW, Barber AG, Challis K, Ranville JF, Single Particle ICP-MS: advances toward routine analysis of nanomaterials, Anal. Bioanal. Chem. 408 (2016) 5053–5074. [DOI] [PubMed] [Google Scholar]
- [134].Laborda F, Bolea E, Jiménez-Lamana J, Single particle inductively coupled plasma mass spectrometry: a powerful tool for nanoanalysis, Anal. Chem. 86 (2014) 2270–2278, 10.1021/ac402980q. [DOI] [PubMed] [Google Scholar]
- [135].Laborda F, Jiménez-Lamana J, Bolea E, Castillo JR, Critical considerations for the determination of nanoparticle number concentrations, size and number size distributions by single particle ICP-MS, J. Anal. At. Spectrom. 28 (2013) 1220–1232. [Google Scholar]
- [136].Jiménez-Lamana J, Marigliano L, Allouche J, Grassl B, Szpunar J, Reynaud S, A novel strategy for the detection and quantification of nanoplastics by single particle inductively coupled plasma mass spectrometry (ICP-MS), Anal. Chem. 92 (2020) 11664–11672. [DOI] [PubMed] [Google Scholar]
- [137].Philippe A, Gangloff M, Rakcheev D, Schaumann GE, Evaluation of hydrodynamic chromatography coupled with inductively coupled plasma mass spectrometry detector for analysis of colloids in environmental media–effects of colloid composition, coating and shape, Anal. Methods 6 (2014) 8722–8728. [Google Scholar]
- [138].Bolea-Fernandez E, Rua-Ibarz A, Velimirovic M, Tirez K, Vanhaecke F, Detection of microplastics using inductively coupled plasma-mass spectrometry (ICP-MS) operated in single-event mode, J. Anal. At. Spectrom. 35 (2020) 455–460. [Google Scholar]
- [139].Rochman CM, Hentschel BT, Teh SJ, Long-term sorption of metals is similar among plastic types: implications for plastic debris in aquatic environments, PLoS One 9 (2014) e85433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Laborda F, Trujillo C, Lobinski R, Analysis of microplastics in consumer products by single particle-inductively coupled plasma mass spectrometry using the carbon-13 isotope, Talanta 221 (2020) 121486. [DOI] [PubMed] [Google Scholar]
- [141].Massos A, Turner A, Cadmium, lead and bromine in beached microplastics, Environ. Pollut. 227 (2017) 139–145. [DOI] [PubMed] [Google Scholar]
- [142].Correia M, Loeschner K, Detection of nanoplastics in food by asymmetric flow field-flow fractionation coupled to multi-angle light scattering: possibilities, challenges and analytical limitations, Anal. Bioanal. Chem. 410 (2018) 5603–5615, 10.1007/s00216-018-0919-8. [DOI] [PubMed] [Google Scholar]
- [143].Sun M, Ye M, Jiao W, Feng Y, Yu P, Liu M, Jiao J, He X, Liu K, Zhao Y, Wu J, Jiang X, Hu F, Changes in tetracycline partitioning and bacteria/phage-comediated ARGs in microplastic-contaminated greenhouse soil facilitated by sophorolipid, J. Hazard. Mater. 345 (2018) 131–139, 10.1016/j.jhazmat.2017.11.036. [DOI] [PubMed] [Google Scholar]
- [144].Lei L, Liu M, Song Y, Lu S, Hu J, Cao C, Xie B, Shi H, He D, Polystyrene (nano) microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans, Environ. Sci. Nano 5 (2018) 2009–2020. [Google Scholar]
- [145].Zhu BK, Fang YM, Zhu D, Christie P, Ke X, Zhu YG, Exposure to nanoplastics disturbs the gut microbiome in the soil oligochaete Enchytraeus crypticus, Environ. Pollut. 239 (2018) 408–415, 10.1016/j.envpol.2018.04.017. [DOI] [PubMed] [Google Scholar]
- [146].Hollóczki O, Gehrke S, Nanoplastics can change the secondary structure of proteins, Sci. Rep. 9 (2019) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Jemec A, Horvat P, Kunej U, Bele M, Kržan A, Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna, Environ. Pollut. 219 (2016) 201–209, 10.1016/j.envpol.2016.10.037. [DOI] [PubMed] [Google Scholar]
- [148].Rehse S, Kloas W, Zarfl C, Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna, Chemosphere 153 (2016) 91–99, 10.1016/j.chemosphere.2016.02.133. [DOI] [PubMed] [Google Scholar]
- [149].Jeong C-B, Kang H-M, Lee M-C, Kim D-H, Han J, Hwang D-S, Souissi S, Lee S-J, Shin K-H, Park HG, Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana, Sci. Rep. 7 (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Jeong C-B, Won E-J, Kang H-M, Lee M-C, Hwang D-S, Hwang U-K, Zhou B, Souissi S, Lee S-J, Lee J-S, Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus), Environ. Sci. Technol. 50 (2016) 8849–8857. [DOI] [PubMed] [Google Scholar]
- [151].Talley KJ, Au SY, Klaine SJ, Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS, The Effect of Microplastic Fibers on the Freshwater Amphipod, Hyalella Azteca, TigerPrints CLEMSON, 2015http://tigerprints.clemson.edu/grads_symposium%0Ahttp://tigerprints.clemson.edu/grads_symposium/199%0Ahttp://tigerprints.clemson.edu/grads_symposium%5Cnhttp://tigerprints.clemson.edu/grads_symposium/199. [Google Scholar]
- [152].Hentschel L, Understanding species-microplastics interactions. A laboratory study on the effects of microplastics on the, (2015) 1–101. [Google Scholar]
- [153].Katzenberger TD, Assessing the biological effects of exposure to microplastics in the three- spined stickleback (Gasterosteus aculeatus) (Linnaeus 1758), University of York, 2015. [Google Scholar]
- [154].Green DS, Boots B, Sigwart J, Jiang S, Rocha C, Effects of conventional and biodegradable microplastics on a marine ecosystem engineer (Arenicola marina) and sediment nutrient cycling, Environ. Pollut. 208 (2016) 426–434, 10.1016/j.envpol.2015.10.010. [DOI] [PubMed] [Google Scholar]
- [155].Chen Q, Gundlach M, Yang S, Jiang J, Velki M, Yin D, Hollert H, Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity, Sci. Total Environ. 584–585 (2017) 1022–1031, 10.1016/j.scitotenv.2017.01.156. [DOI] [PubMed] [Google Scholar]
- [156].Moslehyani A, Ismail AF, Matsuura T, Rahman MA, Goh PS, Recent progresses of ultrafiltration (UF) membranes and processes in water treatment, in: Membr. Sep. Princ. Appl., Elsevier, 2019, pp. 85–110. [Google Scholar]
- [157].Ma B, Xue W, Ding Y, Hu C, Liu H, Qu J, Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment, J. Environ. Sci. (China) 78 (2019) 267–275, 10.1016/j.jes.2018.10.006. [DOI] [PubMed] [Google Scholar]
- [158].Munk P, Aminabhavi TM, Williams P, Hoffman DE, Chmelir M, Some solution properties of polyacrylamide, Macromolecules 13 (1980) 871–876, 10.1021/ma60076a020. [DOI] [Google Scholar]
- [159].Ma B, Xue W, Hu C, Liu H, Qu J, Li L, Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment, Chem. Eng. J. 359 (2019) 159–167. [Google Scholar]
- [160].Judd SJ, The status of industrial and municipal effluent treatment with membrane bioreactor technology, Chem. Eng. J. 305 (2016) 37–45, 10.1016/j.cej.2015.08.141. [DOI] [Google Scholar]
- [161].Kamali M, Appels L, Yu X, Aminabhavi TM, Dewil R, Artificial intelligence as a sustainable tool in wastewater treatment using membrane bioreactors, Chem. Eng. J. 128070 (2020). [Google Scholar]
- [162].Zandi S, Nemati B, Jahanianfard D, Davarazar M, Sheikhnejad Y, Mostafaie A, Kamali M, Aminabhavi TM, Industrial biowastes treatment using membrane bioreactors (MBRs) -a scientometric study, J. Environ. Manage. 247 (2019) 462–473, 10.1016/j.jenvman.2019.06.066. [DOI] [PubMed] [Google Scholar]
- [163].Kamali M, Suhas DP, Costa ME, Capela I, Aminabhavi TM, Sustainability considerations in membrane-based technologies for industrial effluents treatment, Chem. Eng. J. 368 (2019) 474–494, 10.1016/j.cej.2019.02.075. [DOI] [Google Scholar]
- [164].Vo HNP, Bui XT, Nguyen HH, Tran TD, Lee KJ, Nguyen TTT, Dang BT, Bui MH, Nguyen DD, Ngo TTM, Effects of dissolved oxygen concentration on the performance of sponge membrane bioreactor treating hospital wastewater, Desalin. Water Treat. 151 (2019) 128–137, 10.5004/dwt.2019.23828. [DOI] [Google Scholar]
- [165].Lares M, Ncibi MC, Sillanpää M, Sillanpää M, Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology, Water Res. 133 (2018) 236–246, 10.1016/j.watres.2018.01.049. [DOI] [PubMed] [Google Scholar]
- [166].Lv X, Dong Q, Zuo Z, Liu Y, Huang X, Wu WM, Microplastics in a municipal wastewater treatment plant: fate, dynamic distribution, removal efficiencies, and control strategies, J. Clean. Prod. 225 (2019) 579–586, 10.1016/j.jclepro.2019.03.321. [DOI] [Google Scholar]
- [167].Ma J, Wang Z, Xu Y, Wang Q, Wu Z, Grasmick A, Organic matter recovery from municipal wastewater by using dynamic membrane separation process, Chem. Eng. J. 219 (2013) 190–199, 10.1016/j.cej.2012.12.085. [DOI] [Google Scholar]
- [168].Yi Z, Shibin X, Feng H, Dong X, Lingwei K, Zhenbin W, Phosphate removal of acid wastewater from high-phosphate hematite pickling process by in-situ self-formed dynamic membrane technology, Desalin. Water Treat. 37 (2012) 77–83, 10.1080/19443994.2012.661257. [DOI] [Google Scholar]
- [169].Salerno C, Vergine P, Berardi G, Pollice A, Influence of air scouring on the performance of a Self Forming Dynamic Membrane BioReactor (SFD MBR) for municipal wastewater treatment, Bioresour. Technol. 223 (2017) 301–306, 10.1016/j.biortech.2016.10.054. [DOI] [PubMed] [Google Scholar]
- [170].Li L, Xu G, Yu H, Xing J, Dynamic membrane for micro-particle removal in wastewater treatment: performance and influencing factors, Sci. Total Environ. 627 (2018) 332–340. [DOI] [PubMed] [Google Scholar]
- [171].Zhang Z, Su Y, Zhu J, Shi J, Huang H, Xie B, Distribution and removal characteristics of microplastics in different processes of the leachate treatment system, Waste Manag. 120 (2021) 240–247, 10.1016/j.wasman.2020.11.025. [DOI] [PubMed] [Google Scholar]
- [172].Pizzichetti ARP, Pablos C, Álvarez-Fernández C, Reynolds K, Stanley S, Marugán J, Evaluation of membranes performance for microplastic removal in a simple and low-cost filtration system, Case Stud. Chem. Environ. Eng. (2020) 100075. [Google Scholar]
- [173].Poerio T, Piacentini E, Mazzei R, Membrane processes for microplastic removal, Molecules 24 (2019) 4148, 10.3390/molecules24224148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Wang R, Zhang L, Chen B, Zhu X, Low-pressure driven electrospun membrane with tuned surface charge for efficient removal of polystyrene nanoplastics from water, J. Memb. Sci. 614 (2020) 118470, 10.1016/j.memsci.2020.118470. [DOI] [Google Scholar]
- [175].Busse K, Ebner I, Humpf HU, Ivleva N, Kaeppler A, Oßmann BE, Schymanski D, Comment on “Plastic teabags release billions of microparticles and nanoparticles into tea”, Environ. Sci. Technol. 53 (2020) 12300–12310, 10.1021/acs.est.0c03182. [DOI] [PubMed] [Google Scholar]
- [176].Mintenig SM, Bäuerlein PS, Koelmans AA, Dekker SC, Van Wezel AP, Closing the gap between small and smaller: towards a framework to analyse nano- and microplastics in aqueous environmental samples, Environ. Sci. Nano 5 (2018) 1640–1649, 10.1039/c8en00186c. [DOI] [Google Scholar]
- [177].Gigault J, El Hadri H, Reynaud S, Deniau E, Grassl B, Asymmetrical flow field flow fractionation methods to characterize submicron particles: application to carbon-based aggregates and nanoplastics, Anal. Bioanal. Chem. 409 (2017) 6761–6769, 10.1007/s00216-017-0629-7. [DOI] [PubMed] [Google Scholar]
- [178].Schwaferts C, Niessner R, Elsner M, Ivleva NP, Methods for the analysis of submicrometer- and nanoplastic particles in the environment, TrAC - Trends Anal. Chem. 112 (2019) 52–65, 10.1016/j.trac.2018.12.014. [DOI] [Google Scholar]
- [179].Alexy P, Anklam E, Emans T, Furfari A, Galgani F, Hanke G, Koelmans A, Pant R, Saveyn H, Sokull Kluettgen B, Managing the analytical challenges related to micro-and nanoplastics in the environment and food: filling the knowledge gaps, Food Addit. Contam. Part A. 37 (2020) 1–10. [DOI] [PubMed] [Google Scholar]
- [180].da Costa JP, Reis V, Paço A, Costa M, Duarte AC, Rocha-Santos T, Micro (nano) plastics–analytical challenges towards risk evaluation, TrAC Trends Anal. Chem. 111 (2019) 173–184. [Google Scholar]
- [181].Enfrin M, Dumée LF, Lee J, Nano/microplastics in water and wastewater treatment processes – origin, impact and potential solutions, Water Res. 161 (2019) 621–638, 10.1016/j.watres.2019.06.049. [DOI] [PubMed] [Google Scholar]
- [182].Kamali M, Jahaninafard D, Mostafaie A, Davarazar M, Gomes APD, Tarelho LAC, Dewil R, Aminabhavi TM, Scientometric analysis and scientific trends on biochar application as soil amendment, Chem. Eng. J. 395 (2020) 125128, 10.1016/j.cej.2020.125128. [DOI] [Google Scholar]
- [183].Tong M, He L, Rong H, Li M, Kim H, Transport behaviors of plastic particles in saturated quartz sand without and with biochar/Fe3O4-biochar amendment, Water Res. 169 (2020) 115284, 10.1016/j.watres.2019.115284. [DOI] [PubMed] [Google Scholar]