Figure 1: A Disintegrin and A Metalloproteinase Domain 15 (Adam15) expression is induced in the lungs of wild-type (WT) mice exposed to cigarette smoke (CS) and is upregulated on the surface of macrophages, monocytes and CD8+ T cells exposed to CS extract (CSE) in vitro.
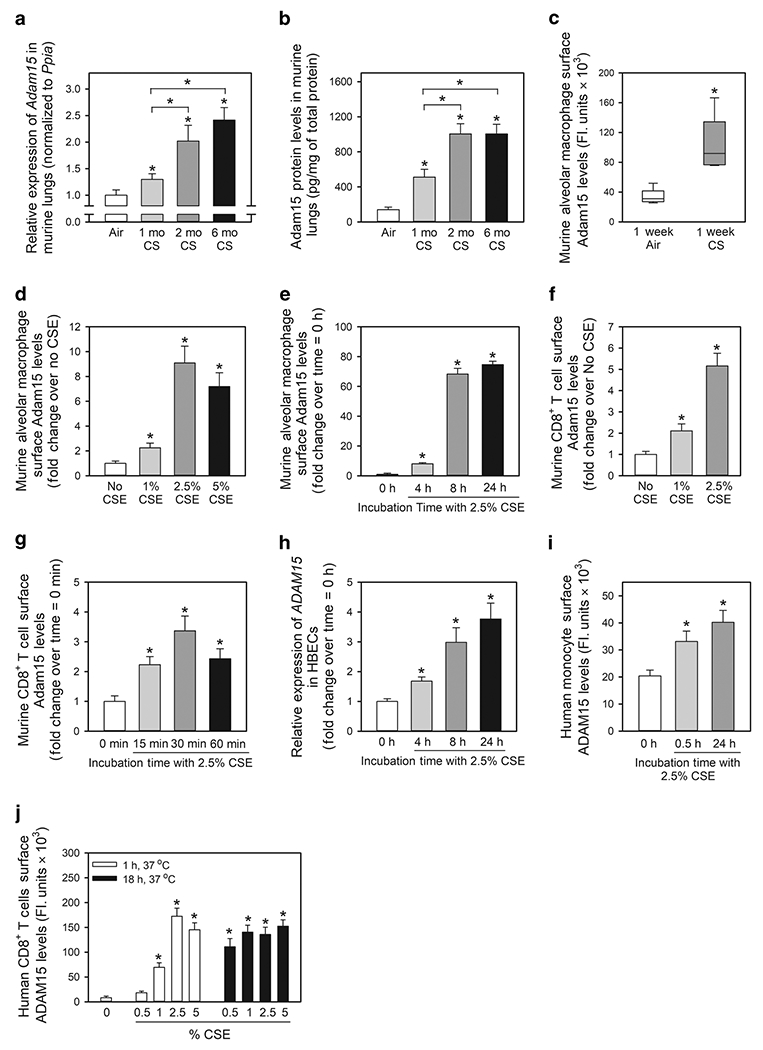
In A and B, Adam15 steady state mRNA and protein levels were measured in the lungs of C57BL/6 WT mice that were exposed to air or CS for up to 6 months using quantitative real-time PCR and an ELISA, respectively (n = 9-10 mice/group). The bar graphs show means ± SD. Data were analyzed using one-way ANOVAs followed by pair-wise testing with two-tailed Student’s t-tests. *, P ≤ 0.004 versus air-exposed mice belonging to the same genotype control or the group indicated. In C, C57BL/6 WT mice were exposed to air or CS for 1 week, and alveolar macrophages (AMs) were isolated using bronchoalveolar lavage. Surface Adam15 protein levels were quantified on AMs using immunostaining techniques, as described in Methods. The boxes in the box-plots show the medians and 25th and 75th percentiles, and the whiskers show the 10th and 90th percentiles; n = 5 mice/group with 200-300 cells analyzed/mouse. Data were analyzed using a Kruskal-Wallis One-Way ANOVA followed by pair-wise testing with Mann-Whitney U tests. *, P = 0.008 versus air group. In D-E, AMs were isolated from naïve WT mice and incubated for 24 h at 37°C with varying concentrations of CS extract (CSE; D) or for varying times with 2.5% CSE (E). Cells were then fixed and immunostained for surface Adam15, as described in Methods. Data are mean ± SEM; n = 150-200 cells/group. The experiments shown are representative of at least three independent experiments yielding similar results. Data were analyzed using a One-Way ANOVA followed by pair-wise testing with two-tailed Student’s t-tests. *, P < 0.001 compared to unstimulated cells. In F-G, CD8+ lymphocytes were isolated from the spleens of naïve WT mice and incubated at 37°C for 2 h with varying concentrations of CSE (F) or with or without 2.5% CSE for varying times up to 60 min (G). Cells were fixed and immunostained for surface Adam15. Data are mean ± SEM; n = 150-200 cells/group. The experiments shown are representative of at least three independent experiments yielding similar results. Data were analyzed using a One-Way ANOVA followed by pair-wise testing with two-tailed Student’s t-tests. *, P < 0.001 compared to unstimulated cells. In H, steady state ADAM15 mRNA levels were measured in normal human bronchial epithelial cells (HBECs) incubated at 37°C for varying times with or without 2.5% CSE using quantitative realtime PCR. Data are mean ± SD; n = 3 experiments. Data were analyzed using a One-Way ANOVA followed by pair-wise testing with two-tailed Student’s t-tests. *, P < 0.03 versus cells incubated without CSE. In I and J, human monocytes and CD8+ T cells were isolated from the blood of healthy donors by positive section for CD14 or CD8α using immunomagnetic beads. Monocytes and CD8+ T cells were incubated with or without CSE for varying times and/or with concentrations of CSE. Cells were fixed and non-permeabilized cells were immunostained for surface ADAM15 as described in Methods. Data are mean ± SEM; n = 150-200 cells/group. The experiments shown are representative of at least three independent experiments yielding similar results. Data were analyzed using a One-Way ANOVA followed by pair-wise testing with two-tailed Student’s t-tests. *, P < 0.001 compared to unstimulated cells.
