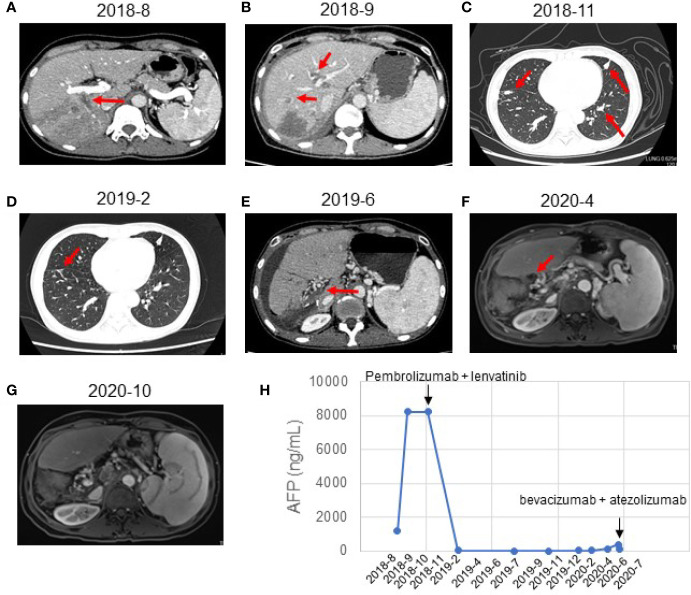
Figure 1.
Response evaluation during the clinical course including changes in imaging and quantitative data. (A) Preoperative abdominal-enhanced CT revealed a mass in the right posterior lobe of the liver (August 21, 2018). (B) CT showed multiple nodules in the liver and tumor thrombus in the right hepatic vein and the left portal vein (September 21, 2018). (C) CT indicated new nodules in the lower lobe of the right lung and the upper lobe of the left lung. The multiple original nodules in the lungs had grown (November 2, 2018). (D) CT indicated a complete response to pembrolizumab plus lenvatinib (February 2019). The pulmonary nodules had disappeared after treatment. (E) CT showed postoperative changes of liver cancer, abnormal density in the liver margin, and a few new inflammatory lesions in both lungs 4 months after treatment with pembrolizumab and lenvatinib (June 2019). (F) MRI showed no local active foci and multiple lymph nodes of different sizes in the hilar area and retroperitoneum (April 2020). (G) MRI showed a complete response to atezolizumab and bevacizumab (October 2020). The retroperitoneal lymph nodes had disappeared after treatment. (H) Graphical depiction of the change in alpha-fetoprotein (AFP) over time.