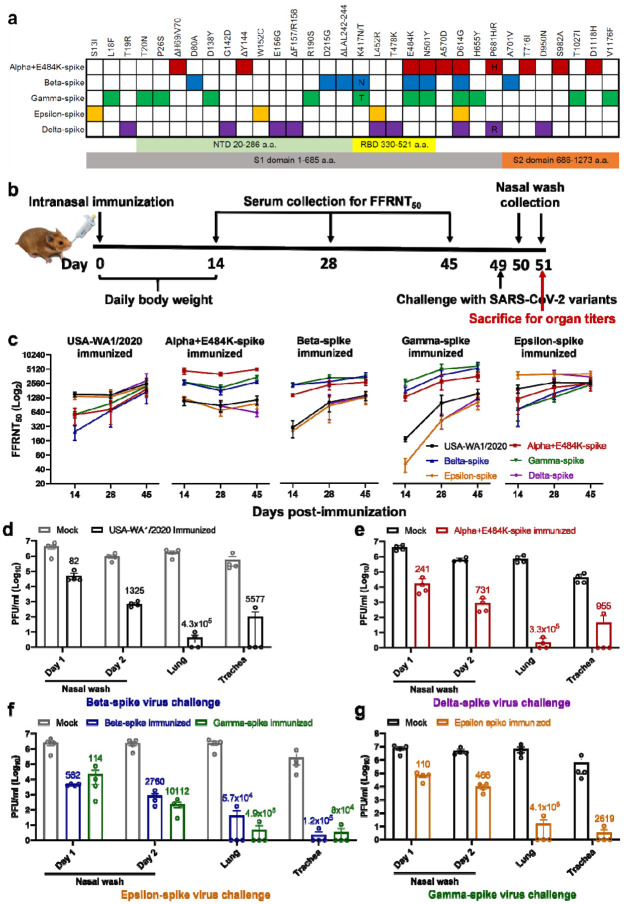
Figure 1. Variant spikes elicit neutralizing antibodies that cross-protect hamsters from challenges with SARS-CoV-2 variants.
a, Amino acid substitutions in the spike protein among SARS-CoV-2 variants. The sequence of the spike from USA-WA1/2020 strain was used as a reference. NTD, N-terminal domain; RBD, Receptor binding domain. b, Experimental scheme of immunization and challenge in hamsters. The hamsters (n=4 per group) were intranasally immunized with 106 PFU of WT or variant-spike SARS-CoV-2. Serum specimens were measured for FFRNT50 values on days 14, 28, and 45 post-immunization. On day 49 post-immunization, the hamsters were intranasally challenged by the indicated variant-spike SARS-CoV-2 (104 PFU). The nasal washes were quantified for viral titers on days 1 and 2 post-challenge. All the hamsters were sacrificed on day 2 post-challenge for viral titers detection. c, Neutralization titers of hamster sera against SARS-CoV-2 spike variants on days 14, 28, and 45 post-immunization. Means ± standard errors of the mean are shown. d-g, Protection of immunized hamsters from the challenge of SARS-CoV-2 spike variants. The immunized hamsters and age-matched non-immunized hamsters (Mock) were challenged with selected variant viruses exhibiting the lowest neutralizing titers. The viral loads in the nasal wash (NW, days 1 and 2), lung, and trachea (day 2) were detected by plaque assays. The numbers above individual columns indicate the fold decrease in viral loads by comparing the means from the immunized group with that from the non-immunized mock group. Means ± standard errors of the mean are shown. The assay limit is 10 PFU/ml.