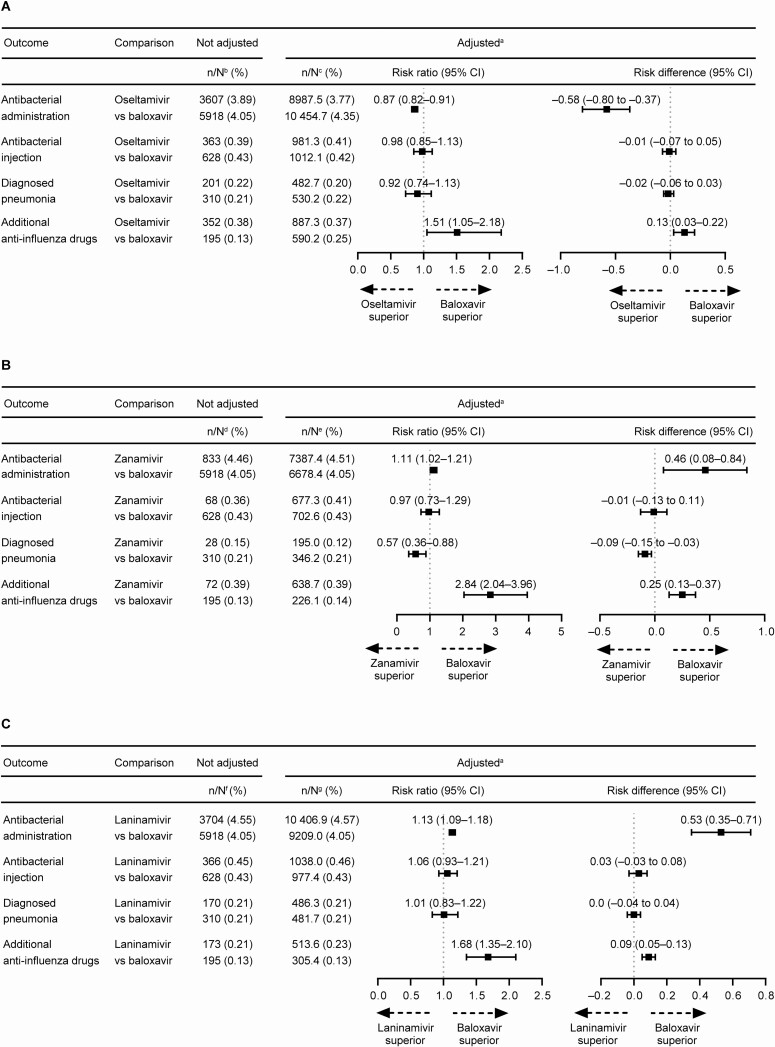
Figure 3.
Risk ratio and risk difference of antibacterial administration (oral and/or injectable), injectable antibacterial use, pneumonia, and additional antiinfluenza drug use regardless of hospitalization. (A) Oseltamivir vs baloxavir. (B) Zanamivir vs baloxavir. (C) Laninamivir vs baloxavir. aData were standardized using the inverse probability of treatment weighting method; the risk ratio was calculated using the exposed (baloxavir) group as the denominator and the neuraminidase inhibitor (NAI) group (oseltamivir, zanamivir, or laninamivir) as the numerator. Risk difference was the incidence of the outcome end point in the NAI group minus the incidence in the baloxavir group. bBaloxavir, N = 146 192; oseltamivir, N = 92 700. cBaloxavir, N = 240 341.4; oseltamivir, N = 238 657.8. dBaloxavir, N = 146 192; zanamivir, N = 18 677. eBaloxavir, N = 164 892.3; zanamivir, N = 163 701.7. fBaloxavir, N = 146 192; laninamivir, N = 81 438. gBaloxavir, N = 227 633.9; laninamivir, N = 227 618.3. Abbreviation: CI, confidence interval.