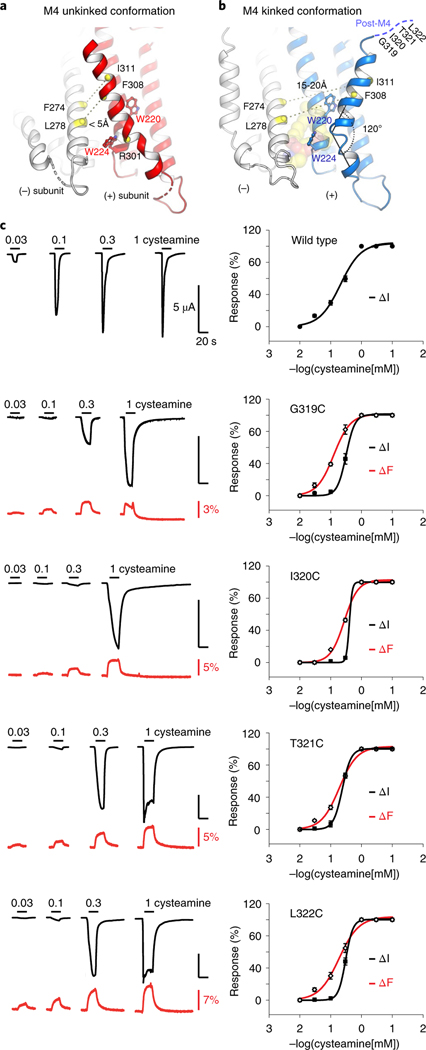
Fig. 5 |. Alternative conformation of an unkinked M4 helix forming novel intersubunit interactions.
a,b, Cartoon representation of the ELIC structure with the M4 helix in an alternative unkinked conformation (red) compared to the original kinked conformation (blue). In the unkinked conformation the M4 helix is pinched between W220 and W224 and sterically hinders lipid interactions. In this conformation residues of the M4 helix, including F308 and I311, come within close range of residues of the M3 helix (white) of its neighboring subunit, including L278 and F274, respectively. In the kinked M4 conformation these residues do not interact (15–20 Å apart). c, VCF of post-M4 ELIC mutants G319C, I320C, T321C and L322C labeled with methanethiosulfonate-rhodamine. Black traces represent cysteamine-induced current responses, red traces represent corresponding changes in fluorescence. Representative traces are shown from five to seven independent experiments for each construct. Panels on the right show normalized current and fluorescence responses as the mean ± s.e.m.