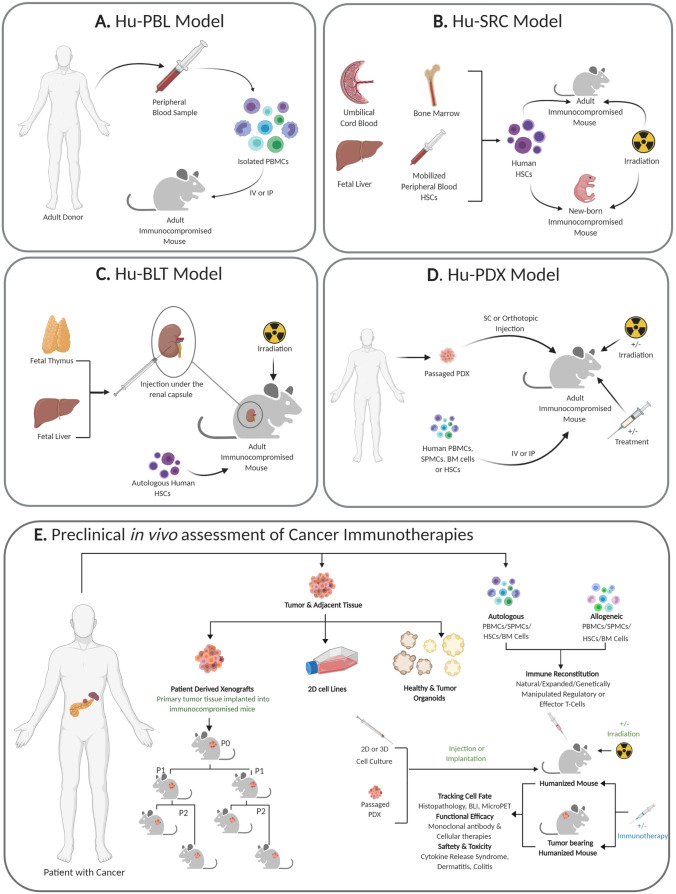
Fig. 2.
Steps involved in establishing human immune system (HIS) models in vivo: a. The hu-PBL (human Peripheral Blood Leukocytes) model can be established through intravenous (IV) or Intraperitoneal (IP) injection of human peripheral blood mononuclear cells (PBMCs) in adult immunocompromised mice. b. The hu-SRC (human Stem Repopulating Cell) model is established through either IV or intrafemoral (IF) injection of haematopoietic stem cells (HSCs) into irradiated adult immunocompromised mice. HSCs are isolated from either umbilical cord blood, bone marrow (BM), fetal liver or mobilized peripheral blood HSCs. Hu-SRC models can also be established through IV, intracardiac (IC) or intrahepatic (IH) injection of HSCs into irradiated newborn immunocompromised mice. c. The hu-BLT (human Bone marrow, Liver, Thymus) model can be established through the transplantation of foetal thymus and liver fragments under the kidney capsule of irradiated adult immunocompromised mice, in addition to the IV injection of autologous HSCs. d. The hu-PDX (HIS patient-derived xenograft) model dually engrafts immunocompromised adult mice with early passage PDXs and a HIS. Most commonly, this is done with the use of immunocompromised mice engrafted with human CD34+ve HSCs but can also be established with human lymphocytes such as PBMC or splenic mononuclear cells (SPMCs). e. Schematic showing the development of preclinical models for the in vivo assessment of cancer immunotherapies. Primary tumor tissue from the patient can be used to derive 2D cell lines and PDOs. A biopsy of primary tissue can also be expanded in vivo in immunocompromised mice to establish a PDX line. Patient derived (autologous) or allogeneic PBMCs, SPMCs or HSCs can be used to generate humanized mice. 2D cells, 3D cells or passaged xenograft tissue can be implanted or injected into the established HIS mice. Both HIS mice and tumor bearing HIS mice can be used to study cell fate, functional efficacy and safety and toxicity of immunotherapies.