Abstract
Background
Paediatric postoperative cerebellar mutism syndrome (ppCMS) is a common complication following the resection of a cerebellar tumour in children. It is hypothesized that loss of integrity of the cerebellar output tracts results in a cerebello-cerebral “diaschisis” and reduced function of supratentorial areas of the brain.
Methods
We performed a systematic review of the literature according to the PRISMA guidelines, in order to evaluate the evidence for hypoperfusion or hypofunction in the cerebral hemispheres in patients with ppCMS. Articles were selected based on the predefined eligibility criteria and quality assessment.
Results
Five studies were included, consisting of three prospective cohort studies, one retrospective cohort study and one retrospective case control study. Arterial spin labelling (ASL) perfusion MRI, dynamic susceptibility contrast (DSC) perfusion MRI and single photon emission computed tomography (SPECT) were used to measure the cerebral and cerebellar tissue perfusion or metabolic activity. Reduced cerebral perfusion was predominantly demonstrated in the frontal lobe.
Conclusions
This systematic review shows that, after posterior fossa tumour resection, cerebral perfusion is reduced in ppCMS patients compared to patients without ppCMS. Well-powered prospective studies, including preoperative imaging, are needed to ascertain the cause and role of hypoperfusion in the pathophysiology of the syndrome.
Keywords: Paediatric postoperative cerebellar mutism syndrome (ppCMS), Posterior fossa syndrome, Cerebral perfusion, Arterial spin labelling (ASL), Dynamic susceptibility contrast (DSC), Single photon emission tomography (SPECT)
Introduction
Paediatric postoperative cerebellar mutism syndrome (ppCMS) is seen in about 23.5% (range 14.7–47.6%) of children after surgery for a cerebellar tumour [1]. The incidence is found to be even higher in medulloblastomas, 39% [2]. The ppCMS is characterized by mutism or a severe reduction in speech, combined with emotional lability and behavioural changes [3]. It generally occurs within 1 week postoperatively and lasts for weeks to years [4].
The most important risk factors for cerebellar mutism are tumour infiltration into the brainstem or vermis, medulloblastoma pathology, tumour size larger than 4 cm and bilateral injury to the dentate-rubro-thalamic tracts [5–8]. These tracts run from the ipsilateral dentate nucleus through the superior cerebellar peduncle, which cross at the level of the mesencephalon to continue towards the contralateral red nucleus and thalamus [6, 9, 10]. The bilateral damage of this tract is thought to result in a so-called cerebello-cerebral diaschisis, a functional disconnection between the cerebellum and cerebrum. The lack of a functional feedback loop may result in hypofunction of supratentorial areas of the brain [11–15].
Imaging studies, i.e. single-photon emission computerized tomography (SPECT), dynamic susceptibility contrast (DSC) and arterial spin labelling (ASL) MRI studies, claim to have demonstrated the occurrence of cerebral hypoperfusion and cerebral metabolic hypofunction in paediatric patients who developed ppCMS after posterior fossa surgery [16–18]. However, these studies are frequently case reports or small case series. Further, in the majority of studies, the imaging analysis is qualitative rather than quantitative.
To evaluate the evidence for hypoperfusion/hypofunction in the cerebral hemispheres in patients with ppCMS, we systematically reviewed the current literature. We aimed to answer the following PICOT question [19]:
How does surgery for a cerebellar tumour (intervention) influence the cerebral blood flow (outcome) in patients with ppCMS (patients), as opposed to patients without ppCMS (comparison), within 4 weeks postsurgery (time)?
Methods
The systematic review is conducted according to the PRISMA guidelines [20]. Studies were identified through searches of electronic databases (PubMed, Embase and Cochrane). The search syntax comprised a combination of synonyms and variables for anatomical location, neuropsychological outcome, participant and perfusion/nuclear imaging (as per 16 March 2021; Table 1). Additional studies were identified by scanning reference lists of included articles. Studies were eligible for inclusion if they met the following criteria:
The study is original.
The domain is children undergoing posterior fossa tumour surgery.
Perfusion or nuclear imaging is used.
Imaging is done within 4 weeks postoperatively
Postoperative neuropsychological assessments are used for the diagnosis of ppCMS.
Table 1.
Search syntax through PubMed and EMBASE
| Anatomical location | Neuropsychological outcome | AND Participants | AND Perfusion/Functional imaging |
|---|---|---|---|
| [ti,ab] | [All fields] | [All fields> | [All fields] |
| "Cerebel*"[tw] OR "posterior fossa surgery"[tw] OR "posterior cranial fossa surgery"[tw] OR "posterior fossa tumor"[tw] OR "posterior fossa tumour"[tw] OR "posterior cranial fossa tumor"[tw] OR "posterior cranial fossa tumour"[tw] OR "posterior fossa tumors"[tw] OR "posterior fossa tumours"[tw] OR "posterior cranial fossa tumors"[tw] OR "posterior cranial fossa tumours"[tw] OR "Cerebellar Neoplasms/surgery"[mesh] OR "Cranial Fossa, Posterior/surgery"[mesh] OR (("Cranial Fossa, Posterior"[mesh] OR "posterior cranial fossa"[tw] OR "posterior fossa"[tw] OR "Cerebellar Neoplasms"[mesh]) AND ("Surgical Procedures, Operative"[Mesh] OR "surgery"[subheading] OR "surgery"[tw] OR "neurosurgery"[tw] OR "surgical*"[tw] OR "resection"[tw] OR "resect*"[tw] OR "neurosurg*"[tw] OR "operation"[tw] OR "operat*"[tw] | "posterior fossa syndrome"[tw] OR "fossa syndrome"[tw] OR "cerebellar mutism syndrome"[tw] OR "mutism syndrome"[tw] OR "Mutism"[Mesh] OR "mutism"[tw] OR "mutism syndrome"[tw] OR "mute"[tw] OR "mutes"[tw] OR "muted"[tw] OR "muting"[tw] OR "oral-verbal expression"[tw] OR "Speech difficult*"[tw] OR "Speech disorder"[tw] OR "Speech defect"[tw] OR "Speech impair*"[tw] OR "language difficult*"[tw] OR "language impair*"[tw] OR "language disorder"[tw] OR "language defect"[tw] OR "diaschisis"[tw] OR "crossed*"[tw] OR "Speech Disorders"[mesh] OR "Speech Sound Disorder"[Mesh] OR "speech"[tw] OR "speech*"[tw] OR "language"[tw] OR "speaking"[tw] OR "speak*"[tw | "Child*"[tw] OR "child"[mesh] OR "infant*"[tw] OR "pediatric*"[tw] OR "paediatric*"[tw] OR "pediatrics"[mesh] OR "girl"[tw] OR "girls"[tw] OR "girlhood"[tw] OR "boy"[tw] OR "boy"[tw] OR "boyhood"[tw] | "Arterial spin labeling"[tw] OR "ASL"[tw] OR "Spin Labels"[Mesh] OR "perfusion weighted mri"[tw] OR "PWI"[tw] OR "perfusion weighted imag*"[tw] OR "Perfusion Imaging"[Mesh] OR "Perfusion Imaging"[tw] OR "dynamic susceptibility contrast"[tw] OR "DSC"[tw] OR "mr perfusion"[tw] OR "Magnetic Resonance Perfusion"[tw] OR "Magnetic Resonance Angiography"[Mesh] OR "dynamic contrast enhanced"[tw] OR "DCE"[tw] OR "hypoperfusion"[tw] OR "decreased perfusion"[tw] OR "altered perfusion"[tw] OR "frontal lobe perfusion"[tw] OR "temporal lobe perfusion"[tw] OR "parietal lobe perfusion"[tw] OR "occipital lobe perfusion"[tw] OR "cerebral perfusion"[tw] OR "frontal lobe hypoperfusion"[tw] OR "temporal lobe hypoperfusion"[tw] OR "parietal lobe hypoperfusion"[tw] OR "occipital lobe hypoperfusion"[tw] OR "Tomography, Emission-Computed, Single-Photon"[Mesh] OR "SPECT"[tw] OR "Single Photon Emission Tomography"[tw] OR "functional mri"[tw] OR "fmri"[tw] OR "functional magnetic"[tw] OR "Magnetic Resonance Imaging"[Mesh] OR "Blood flow"[tw] OR "blood oxyg*"[tw] OR "hemodynamic response"[tw] OR "bold contrast"[tw] OR "Blood-oxygen level dependent contrast"[tw] OR"Neuroimaging"[Mesh] OR "neuroimag*"[tw] OR "brain ischemia"[mesh] |
Study selection
Articles were independently screened by two reviewers (NA, KvB), first on title and abstract, and finally on full text to determine eligibility. Disagreements between the two reviewers were resolved by discussion after reading full text.
Risk of bias evaluation
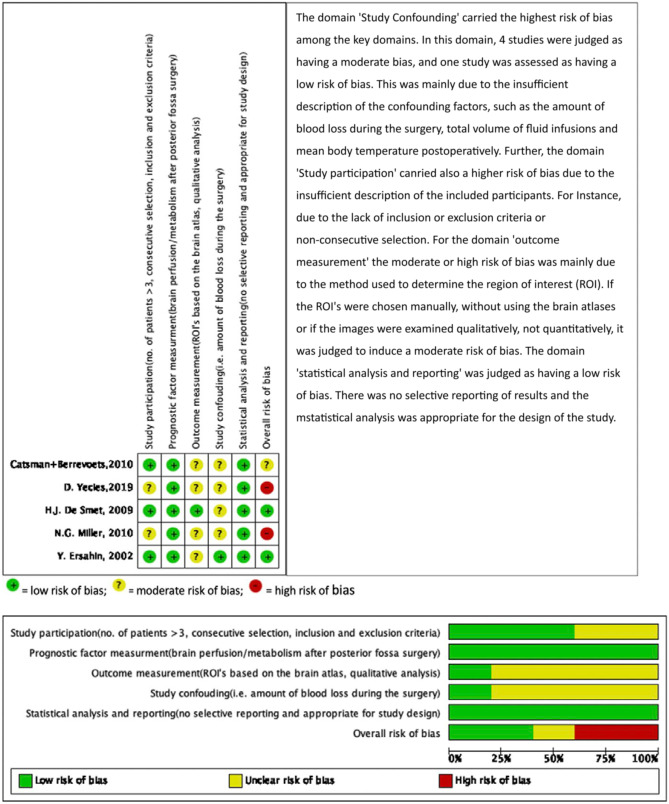
The risk of bias in the individual studies was assessed by two reviewers (NA, KvB) using the Quality in Prognosis Studies tool (QUIPS) developed by Cochrane [35]. The QUIPS tool was based on the following key domains: study participation, prognostic factor measurement, outcome measurement, study confounding, statistical analysis and reporting. The overall risk of bias was judged as low, moderate or high based on the following criteria: (1) low if there was a low risk of bias in all key domains, or only one unclear risk of bias in one key domain, (2) unclear/moderate risk of bias if there was an unclear risk of bias for two key domains and (3) high risk of bias if there was a high risk of bias for one or more key domains or unclear risk of bias for more than two key domains.
Data extraction
Data were extracted from the studies on the number of included patients (with and without ppCMS), patient age and gender, tumour location, type of pathology, type of perfusion/nuclear imaging, time interval between surgery and postoperative scan, postoperative neuropsychological tests and outcome measures (e.g. mean deviation (MD) with 95% confidence interval (CI)). If the data were not reported, outcome measures were calculated based on other reported data or based on the qualitative assessment of the nuclear specialist. The standard unit for measurement of cerebral blood flow (CBF) is ml blood /min/100 g tissue [21]. As there are no cut-off values for normal cerebral perfusion in children, the definition of hypoperfusion was at the discretion of the authors.
Any data on possible confounding factors, such as the amount of blood loss during the surgery, total volume of fluid infusions and mean body temperature, were also collected [8].
Results
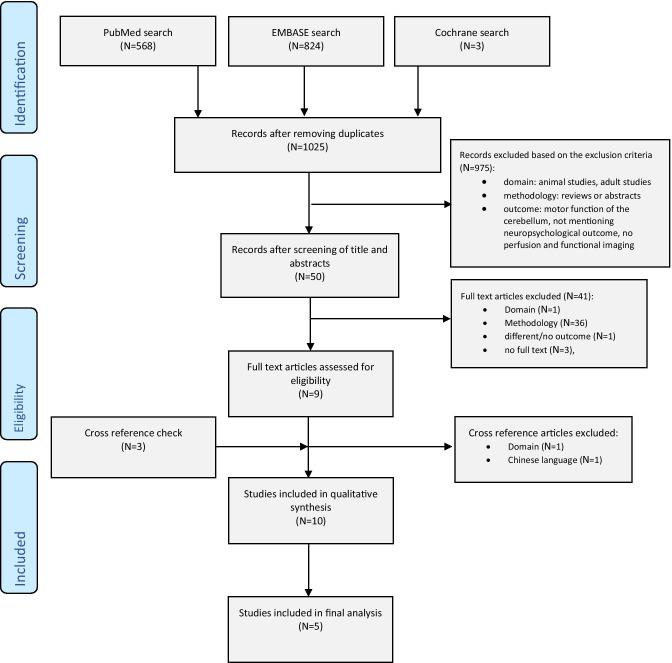
The search identified 1125 articles from PubMed and Embase databases, of which 50 fulfilled the inclusion criteria, based on title and abstract. One study was found through cross reference checking (Fig. 1). After screening full text, five studies were included in this review, consisting of three prospective cohort studies, one retrospective cohort study and one retrospective case control study (Table 2).
Fig. 1.
PRISMA Flow Diagram
Table 2.
Baseline characteristics of patients in the selected articles
| Authors | Study design | PreopImaging* | PostopImaging* | Time to Postop imaging(weeks) | ppCMS + | ppCMS - | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age(mean) | Gender(M/F) | Tumour location | Pathology | N. | Age(mean) | Gender(M/F) | Tumour location | Pathology | |||||
| Ersahin et al. [24] | PS C | - | SPECT | 1 | 3 | 3.3 | 1M/2F | 4V, V | 2MB,1EP | 11 | 6.6 | 5M/6F | V, 4V, CH | 5PA, 3EP, 1MB, 1DT, 1CPP |
| De Smet et al. [22] | PS C | - | SPECT | 1-4 | 4 | 7.5 | 4M | V | 2MB, 1PA, 1EP | 1 | 14 | 1M | V | 1PA |
| Catsman-Berrevoets et al. [25] | PS C | - | SPECT | 1-4 | 13 | 9.3 | 9M/4F | V | 3PA, 10MB | 2 | 8.5 | 2M | V | 2MB |
| Yecies et al. [23 | RS C | - | ASL | 1 | 14 | 7.1 | 8M/6F | 4V, V | 14MB | 10 | NM | NM | 4V, V | 10MB |
| Miller et al. [26] | RS CC | - | DSC | 3-4 | 11 | 7.1 | NM | NM | MB, ATRT or PN | 11 | 7.33 | NM | NM | NM |
N number of subjects, NM not mentioned, NA not applicable, M male, F female RS retrospective, PS prospective, CR case report, CC case control, C cohort. ASL Arterial Spin Labelling, DSC Dynamic Susceptibility Contrast, SPECT Single Photon Emission Computed Tomography
PA pilocytic astrocytoma, MB medulloblastoma, EP anaplastic ependymoma, ATRT atypical teratoid/rhabdoid, PN primitive neuroectodermal, DT dermoid tumour, SB spongioblastoma, CPP choroid plexus papilloma
V vermis, 4V fourth ventricle, LC left cerebellar, CH cerebellar hemisphere
* nuclear or perfusion imaging
The baseline characteristics of the patients included in these five studies are shown in Table 2. The total number of patients was 80. Age was reported with a median of 7 years (range 2–18 years). In the majority of the studies, more boys were included than girls. One study used ASL perfusion MRI, one study used DSC perfusion MRI and three other studies used SPECT to measure the cerebral and cerebellar tissue perfusion/metabolic activity. The time to postoperative imaging follow-up was within 4 weeks. Pre-operative perfusion/nuclear imaging was only acquired in one out of a total of 80 patients [22]. Medulloblastoma was the most frequent tumour pathology among the patients, in the ppCMS as well as the non-ppCMS group, and tumours were mainly located in the fourth ventricle and the cerebellar vermis.
Based on the evaluation of 6 key domains, 3 studies were judged to have a low risk of bias and 2 studies had a high risk of bias. This was largely due to the fact that there was a lack of information on patient selection, no correction for confounding factors and heterogeneity in outcome measurements (Fig. 2). Cerebral perfusion was evaluated using different imaging and software techniques with a large variability in outcome measures. For instance, in the ASL study of Yecies et al., the Regions of Interest (ROIs) were acquired manually without using atlases [23]. Further, in some studies, the CBF measurements were acquired qualitatively, not quantitatively; hence, the measurements might have been subject to the investigator’s interpretation [24].
Fig. 2.
Risk of bias according to the QUIPS tool
Most of the studies assessed brain tissue perfusion in the following anatomical areas: frontal lobe, parietal lobe, temporal lobe, occipital lobe, thalamus, cerebellum and proximal efferent cerebellar pathways (Table 3). Brain perfusion in the corpus cinguli and brainstem was evaluated in only one study.
Table 3.
Perfusion of different brain regions
| ppCMS + | ppCMS - | ||||||||||||||||
| Author | Imaging | CBF | FRO | PAR | TEM | OCC | CER | THA | Other | CBF | FRO | PAR | TEM | OCC | CER | THA | Other |
| Ersahin et al., 200224 | SPECT | NA | B- | B- | n | n | R- | n | NA | NA | n | n | n | n | n | n | NA |
| De Smet et al., 200922 | SPECT | NA | B- | B- | B- | R- | B- | B- | L- CC | NA | R- | n | n | L- | L- | n | n |
| Catsman-Berrevoets et al., 201025 | SPECT | NA | B- | n | B- | B- | B- | n | BS- | NA | n | n | n | n | n | n | n |
| Yecies et al., 201923 | ASL | R:37.00 ±13.46, L: 41.00 ± 17.36 | B- | n | n | n | n | n | NA | R:49.70 ± 13.72, L: 53.50 ± 14.62 | n | n | n | n | n | n | NA |
| Miller et al., 201026 | DSC | 47.3 to 54.5 | B- | R- | R- | n | n | n | NA | 55.9 to 67.6 | n | n | n | n | n | n | NA |
NA not applicable, NM not mentioned, ASL Arterial Spin Labelling, DSC Dynamic Susceptibility Contrast, SPECT Single Photon Emission Computed Tomography, R right, L left, B bilateral, CBF mean cerebral blood flow in ml/min/100g, CER cerebellum, THA thalamus, FRO frontal, PAR parietal, TEM temporal, OCC occipital , CC corpus cinguli, BS brainstem, n normal
In all included studies, hypoperfusion was demonstrated almost exclusively in children with ppCMS when compared to controls. Reduced cerebral perfusion was predominantly demonstrated not only in the frontal lobe, but also in the temporal lobe, parietal lobe, occipital lobe, thalamus and the cerebellum. Hypoperfusion was reported to occur either bilaterally or in the right hemisphere, except in one study that showed hypoperfusion in the left cingulate gyrus in the language-dominant left or bilateral frontal lobe and in the left cingulate gyrus during the mutism phase [22].
The quantitative analysis of Yecies et al. demonstrated a significant difference in right frontal lobe perfusion in ppCMS patients compared to the controls (mean deviation −12.70 [CI −23.75 to −1.65], p = 0.046) (Table 3) [23]. There was also hypoperfusion in the left frontal lobe (mean deviation −12.50 [CI −25.34 to 0.34], p = 0.092) in ppCMS patients as compared to the controls, but this difference was not statistically significant.
Increased perfusion, or hyper-perfusion, was not reported by any of the studies.
Discussion
This systematic review shows that, after posterior fossa tumour resection, cerebral perfusion is reduced in ppCMS patients compared to patients without ppCMS. All included studies show predominantly bilateral frontal lobe hypoperfusion in children with ppCMS.
ASL perfusion imaging identified decreased frontal lobe blood flow in the immediate postoperative period in ppCMS group as compared to non-ppCMS patients. In addition, this hypoperfusion resolved after the period of mutism [23].
The DSC study showed a decrease in cerebral blood flow within frontal, right parietal and temporal areas. Further, a global cerebral cortical hypoperfusion is seen in the ppCMS group as compared to controls [26]. Only one study found hypoperfusion in the non-ppCMS group, but in different regions and only in one patient [22].
Although case reports were excluded from our systematic analysis, they do support our findings. All case reports demonstrated hypoperfusion of the frontal lobes bilaterally [16, 18, 27]. Further, Sagiuch et al. and Watanabe et al. reported additional hypoperfusion bilaterally in the cerebellum and the thalami. Sagiuch et al. and Germano et al. also reported a reduced cerebral perfusion in the language dominant left temporal lobe. The clinical improvement after the period of mutism coincided with improvement in SPECT and ASL images [16, 18].
Imaging modalities
Arterial spin labelling (ASL) has several advantages when compared to DSC and SPECT imaging. ASL is a magnetic resonance imaging technique that enables the measurements of cerebral blood flow non-invasively by magnetically labelling inflowing blood at the tissue level [28, 29]. It does not require the administration of an intravenous contrast agent and is thus easily repeatable. It does require a high signal to noise ratio, which is better in children compared to adults because of their higher cerebral blood flow [31, 32].
Contrary to ASL, both DSC and SPECT imaging are invasive imaging techniques for both adult and paediatric patients. SPECT requires intravenous administration of technetium-99 m-labelled d, l-hexamethyl propylene amine oxime (Tc99M-HM-PAO), and DSC imaging requires bolus intravenous injection of a gadolinium-based contrast agent for the measurement of its regional cerebral uptake [11, 16]. Therefore, ASL is the technique of choice for (repeated) perfusion measurement in young children [29, 30].
Limitations
None of the included studies in this review conducted pre-operative perfusion or nuclear imaging, in order to compare with the postoperative findings. Preoperative comparison is important for a correct interpretation of the postoperative findings. For instance, hematocrit effects in patients with anaemia can confound the interpretation of CBF changes measured using ASL MRI [33]. More importantly, a perfusion deficit that was already present preoperatively cannot be ascribed to the surgery and cannot explain the pathophysiology of the postoperative cerebellar mutism syndrome.
Pre-operative perfusion imaging was conducted in only one case report [18]. In this single case, postoperative ASL perfusion MRI revealed hypoperfusion bilaterally in the frontal lobes, the thalamus and the cerebellar hemispheres when compared to preoperative ASL [18].
Further, none of the studies mentioned any confounding factors. A higher mean body temperature on the first postoperative days might increase metabolic stress, while the area of surgery and the brain tissue surrounding the site of surgery is in an already critical metabolic status [8]. Further, fluid balance, including volume of blood loss and total volume of liquid infusions, might affect the CBF [8].
Another limitation was the large heterogeneity among the included studies that may have influenced the results of this systematic review.
Level of evidence
According to the Oxford Centre for Evidence Based Medicine [34], this systematic review offers a “Level 4” evidence despite the systematic approach and the strict selection of articles.
Clinical importance
Even though there seems to be convincing evidence for bilateral frontal perfusion deficits in ppCMS patients as compared to non-ppCMS patients, without a preoperative comparison, this does not fully support the hypothesis of cerebello-cerebral diaschisis as pathophysiological explanation of the syndrome. Recognizing the ppCMS and understanding its pathophysiology is important for education of patients and families, to develop treatment strategies and rehabilitation programs and, eventually, to prevent its occurrence.
Future research should aim for a large prospective observational cohort study including non-invasive imaging techniques such as ASL both pre- and postoperatively, allowing quantitative measurement of the CBF in children with and without ppCMS. This will give valuable information to further unravel the pathophysiology of this devastating syndrome.
Conclusion
This systematic analysis of the literature shows that bilateral frontal lobe hypoperfusion is predominantly seen in patients with ppCMS as compared to those without. Well-powered prospective studies, including preoperative imaging, are needed to ascertain the cause and role of hypoperfusion in the pathophysiology of the syndrome.
Declarations
Conflict of interest
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Narjes Ahmadian, Email: n.ahmadian786@gmail.com.
K. M. van Baarsen, Email: K.M.vanBaarsen-4@prinsesmaximacentrum.nl
P. A. J. T. Robe, Email: P.Robe@umcutrecht.nl
E. W. Hoving, Email: E.W.Hoving-3@prinsesmaximacentrum.nl
References
- 1.Renne B, Radic J, Agrawal D, et al. Cerebellar mutism after posterior fossa tumor resection in children: a multicenter international retrospective study to determine possible modifiable factors. Child’s Nerv Syst. 2020;36(6):1159–1169. doi: 10.1007/s00381-019-04058-7. [DOI] [PubMed] [Google Scholar]
- 2.Wells EM, Khademian ZP, Walsh KS, et al. Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: neuroradiographic features and origin. J Neurosurg Pediatr. 2010;5(4):329–334. doi: 10.3171/2009.11.PEDS09131. [DOI] [PubMed] [Google Scholar]
- 3.Gudrunardottir T, Morgan AT, Lux AL, et al. Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Child’s Nerv Syst. 2016;32(7):1195–1203. doi: 10.1007/s00381-016-3093-3. [DOI] [PubMed] [Google Scholar]
- 4.Sergeant A, Kameda-Smith MM, Manoranjan B, et al. Analysis of surgical and MRI factors associated with cerebellar mutism. J Neurooncol. 2017;133(3):539–552. doi: 10.1007/s11060-017-2462-4. [DOI] [PubMed] [Google Scholar]
- 5.Ojemann JG, Partridge SC, Poliakov AV, et al. Diffusion tensor imaging of the superior cerebellar peduncle identifies patients with posterior fossa syndrome. Child’s Nerv Syst. 2013;29(11):2071–2077. doi: 10.1007/s00381-013-2205-6. [DOI] [PubMed] [Google Scholar]
- 6.Küper M, Timmann D. Brain & Language Cerebellar mutism. Brain Lang [Internet] 2013;127(3):327–333. doi: 10.1016/j.bandl.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Catsman-Berrevoets CE, Van Dongen HR, Mulder PGH, et al. Tumour type and size are high risk factors for the syndrome of “cerebellar” mutism and subsequent dysarthria. J Neurol Neurosurg Psychiatry. 1999;67(6):755–757. doi: 10.1136/jnnp.67.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pols SYCV, Van Veelen MLC, Aarsen FK, et al. Risk factors for development of postoperative cerebellar mutism syndrome in children after medulloblastoma surgery. J Neurosurg Pediatr. 2017;20(1):35–41. doi: 10.3171/2017.2.PEDS16605. [DOI] [PubMed] [Google Scholar]
- 9.Van Baarsen KM, Grotenhuis JA. The anatomical substrate of cerebellar mutism. Med Hypotheses [Internet] 2014;82(6):774–780. doi: 10.1016/j.mehy.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Toescu SM, Hettige S, Phipps K, et al. Post-operative paediatric cerebellar mutism syndrome: time to move beyond structural MRI. Child’s Nerv Syst. 2018;34(11):2249–2257. doi: 10.1007/s00381-018-3867-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patay Z. Postoperative posterior fossa syndrome: unraveling the etiology and underlying pathophysiology by using magnetic resonance imaging. Child’s Nerv Syst [Internet] 2015;31(10):1853–1858. doi: 10.1007/s00381-015-2796-1. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Guan M, Lian HJ, et al. Crossed cerebellar diaschisis detected by arterial spin-labeled perfusion magnetic resonance imaging in subacute ischemic stroke. J Stroke Cerebrovasc Dis [Internet] 2014;23(9):2378–2383. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Pollock JM, Tan H, Kraft RA, et al. Arterial spin-labeled MR perfusion imaging: clinical applications. Magn Reson Imaging Clin N Am [Internet] 2009;17(2):315–338. doi: 10.1016/j.mric.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusano Y, Tanaka Y, Takasuna H, et al. Transient cerebellar mutism caused by bilateral damage to the dentate nuclei after the second posterior fossa surgery: case report. J Neurosurg. 2006;104(2):329–331. doi: 10.3171/jns.2006.104.2.329. [DOI] [PubMed] [Google Scholar]
- 15.Koh S, Turkel SB, Baram TZ (1997) Cerebellar mutism in children: report of six cases and potential mechanisms. Pediatr Neurol 16:218–21 [DOI] [PMC free article] [PubMed]
- 16.Germanò A, Baldari S, Caruso G, et al. Reversible cerebral perfusion alterations in children with transient mutism after posterior fossa surgery. Child’s Nerv Syst. 1998;14(3):114–119. doi: 10.1007/s003810050191. [DOI] [PubMed] [Google Scholar]
- 17.Catsman-Berrevoets CE, Van Breemen M, Van Veelen ML, et al. Supratentorial arterial ischemic stroke following cerebellar tumor resection in two children. Pediatr Neurosurg. 2005;41(4):206–211. doi: 10.1159/000086563. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe Y, Yamasaki F, Nakamura K, et al. Evaluation of cerebellar mutism by arterial spin-labeling perfusion magnetic resonance imaging in a patient with atypical teratoid/rhabdoid tumor (AT/RT): a case report. Child’s Nerv Syst. 2012;28(8):1257–1260. doi: 10.1007/s00381-012-1741-9. [DOI] [PubMed] [Google Scholar]
- 19.Riva JJ, Malik KMP, Burnie SJ, et al. What is your research question? An introduction to the PICOT format for clinicians. J Can Chiropr Assoc [Internet] 2012;56(3):167–171. [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M et al (2015) Evaluation of ASTM standard test method E 2177, 6 retroreflectivity of pavement markings in a condition of 7 wetness. Syst Rev :1–9
- 21.Mutsaerts HJMM, Petr J, Václavů L, et al. The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. J Cereb Blood Flow Metab. 2017;37(9):3184–3192. doi: 10.1177/0271678X16683690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Smet HJ, Baillieux H, Wackenier P, et al. Long-term cognitive deficits following posterior fossa tumor resection: a neuropsychological and functional neuroimaging follow-up study. Neuropsychology. 2009;23(6):694–704. doi: 10.1037/a0016106. [DOI] [PubMed] [Google Scholar]
- 23.Yecies D, Shpanskaya K, Jabarkheel R, et al. Arterial spin labeling perfusion changes of the frontal lobes in children with posterior fossa syndrome. J Neurosurg Pediatr. 2019;24(4):382–388. doi: 10.3171/2019.5.PEDS18452. [DOI] [PubMed] [Google Scholar]
- 24.Erşahin Y, Yararbas Ü, Duman Y, et al. Single photon emission tomography following posterior fossa surgery in patients with and without mutism. Child’s Nerv Syst. 2002;18(6–7):318–325. doi: 10.1007/s00381-002-0614-z. [DOI] [PubMed] [Google Scholar]
- 25.Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the posterior fossa syndrome in children after cerebellar tumour surgery. Cortex [Internet] 2010;46(7):933–946. doi: 10.1016/j.cortex.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Miller NG, Reddick WE, Kocak M, et al. Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. Am J Neuroradiol. 2010;31(2):288–294. doi: 10.3174/ajnr.A1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sagiuchi T, Ishii K, Aoki Y, et al. Bilateral crossed cerebello-cerebral diaschisis and mutism after surgery for cerebellar medulloblastoma. Ann Nucl Med. 2001;15(2):157–160. doi: 10.1007/BF02988609. [DOI] [PubMed] [Google Scholar]
- 28.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM Perfusion Study Group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biagi L, Abbruzzese A, Bianchi MC, et al. Age dependence of cerebral perfusion assessed by magnetic resonance continuous arterial spin labeling. J Magn Reson Imaging. 2007;25(4):696–702. doi: 10.1002/jmri.20839. [DOI] [PubMed] [Google Scholar]
- 30.Haller S, Zaharchuk G, Thomas DL, et al. Arterial spin labeling perfusion of the brain. Radiology [Internet] 2016;28(2):337–356. doi: 10.1148/radiol.2016150789. [DOI] [PubMed] [Google Scholar]
- 31.Ferré JC, Bannier E, Raoult H, et al. Arterial spin labeling (ASL) perfusion: techniques and clinical use. Diagn Interv Imaging [Internet] 2013;94(12):1211–1223. doi: 10.1016/j.diii.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Grade M, Hernandez Tamames JA, Pizzini FB, et al. A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology. 2015;57(12):1181–1202. doi: 10.1007/s00234-015-1571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu HS, Jawad AF, Laney N, et al. Effect of blood T1 estimation strategy on arterial spin labeled cerebral blood flow quantification in children and young adults with kidney disease. J Neuroradiol [Internet] 2019;46(1):29–35. doi: 10.1016/j.neurad.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cebm (2009) CEBM. [Online]. Available from: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed 12 July 2020
- 35.Hayden JA, Van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]