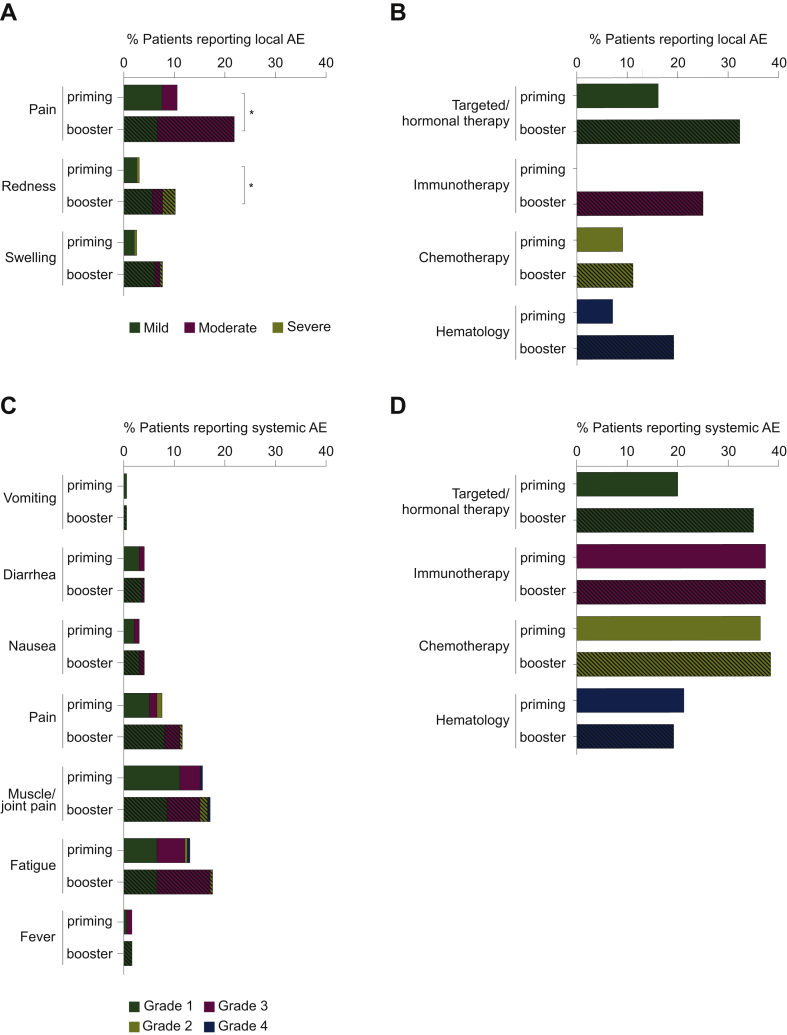
Figure 2.
Local and systemic adverse events (AEs) reported within 7 days after priming and booster BNT162b2 vaccination.
(A) Local and (C) systemic AEs have been pooled from all study cohorts and are represented as a percentage of the total study population (n = 200 for priming vaccination, n = 197 for booster vaccination). To show differences in the occurrence of AEs between study cohorts, the proportion of patients reporting AEs are represented as a percentage of the number of patients in that cohort (first vaccine dose: hematology cohort, n = 41; targeted/hormonal therapy cohort, n = 80; chemotherapy cohort, n = 63; immunotherapy cohort, n = 16; second vaccine dose: hematology cohort, n = 41; targeted/hormonal therapy cohort, n = 80; chemotherapy cohort, n = 60; immunotherapy cohort, n = 16) (B and D). Open bars represent the AEs reported after the first vaccine dose while the dashed bars represent the ones reported after the second vaccine dose. Comparisons between both vaccine administrations were performed using a McNemar test with Bonferroni-Holm correction for the number of cohorts (n = 4) and the number of different local AEs (n = 3) and systemic AEs (n = 7) are reported: ∗P < 0.05.