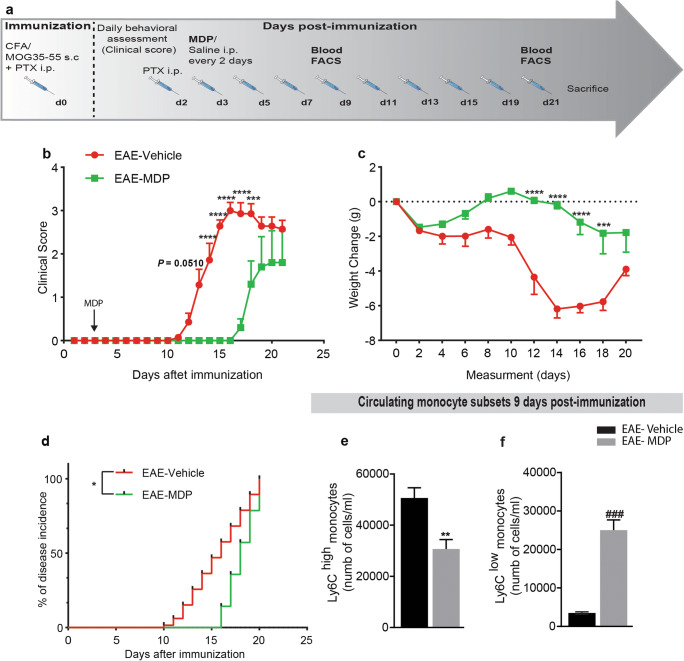
Fig. 3.
Mice were highly resistant to EAE onset via shifting monocyte subsets towards Ly6Clow monocytes in response to the MDP treatment. (a) Representative timeline of the entire protocol, including EAE inducing, MDP administrations, and FACS analysis in mice treated with vehicle (n = 7) or MDP (n = 7). (b) Clinical scores of mice treated with vehicle or MDP were determined daily after immunization. Data are expressed as the means ± SEM; ****P < 0.0001, ***P = 0.0008, two-way ANOVA followed by Tukey’s multiple comparisons test. (c) The variation of body weight has been expressed compared to the day of EAE induction (day 0) as mean ± SEM; ****P < 0.0001, ***P = 0.0001, two-way ANOVA followed by Tukey’s multiple comparisons test. (d) Percentage of disease incidence in mice treated with vehicle or MDP. Data are expressed as the means ± SEM; *P = 0.02, log–rank (Mantel–Cox) test. (e) and (f) Absolute count of blood Ly6Chi and Ly6Clow monocytes respectively following treatment with vehicle or MDP in EAE mice as measured by flow cytometry 1 week after MDP injections (9 days after immunization). Data are expressed as the means ± SEM; **P < 0.05 versus EAE–vehicle, ###P < 0.0001 versus EAE–vehicle, Student’s t test