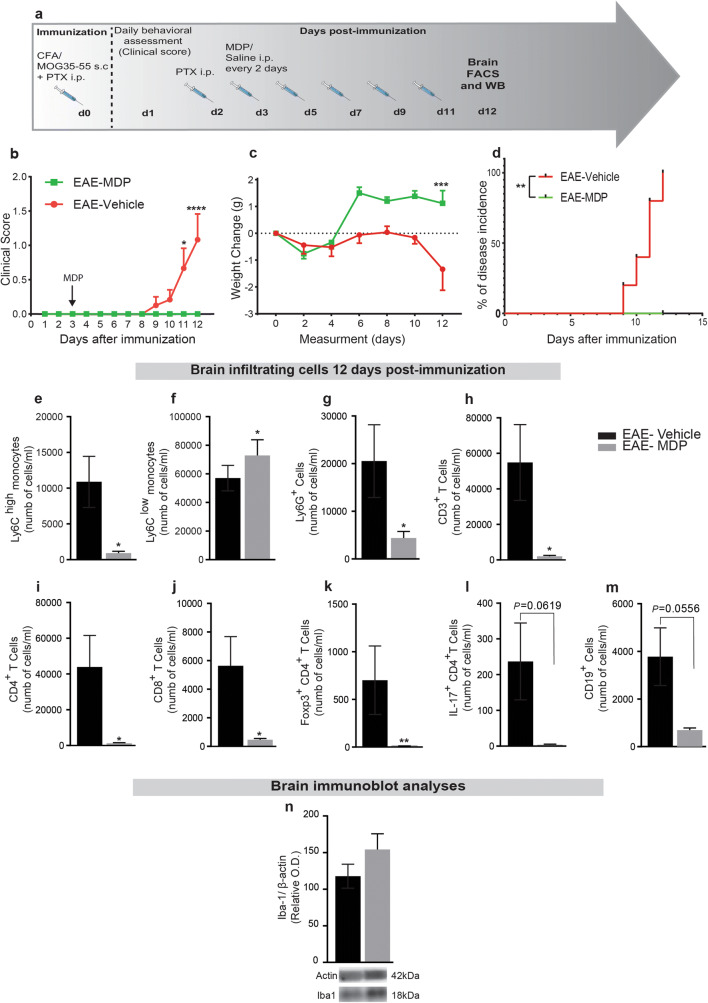
Fig. 4.
MDP modulates monocyte subsets and infiltrating Ly6Chi, Ly6Clow monocytes, T-cell subsets, Ly6G+ cells, and CD19+ cells in the CNS before the onset of EAE. (a) Representative timeline of the entire protocol, including inducing EAE, MDP administrations, and FACS analysis in WT mice treated with vehicle (n = 10) or MDP (n = 9). (b) Clinical scores of mice in the groups were determined daily after immunization. Data are expressed as the means ± SEM; *P = 0.03, ****P < 0.0001, two-way ANOVA followed by Tukey’s multiple comparisons test. (c) Body weight changes measured after immunization in the treatment and control groups. Data are expressed as the means ± SEM; ***P = 0.0001, two-way ANOVA followed by Tukey’s multiple comparisons test. (d) Percentage of disease incidence in mice treated with vehicle or MDP. Data are expressed as the means ± SEM; **P = 0.002, log–rank (Mantel–Cox) test. (e) and (f) Absolute count of CNS Ly6Chi and Ly6Clow monocytes respectively following treatment with vehicle or MDP in EAE mice as measured by FACS 12 days after immunization. Data are expressed as the means ± SEM; *P < or = 0.02, Student’s t test. (g) Absolute count of CNS Ly6G+ cells following treatment in the experimental groups. Data are expressed as the means ± SEM; *P < or = 0.02, Student’s t test. (h)–(j) Absolute count of CNS CD3+, CD4+, and CD8+ T cells respectively following treatment in the experimental EAE groups measured by FACS. Data are expressed as the means ± SEM; *P < or = 0.04, Student’s t test. (k) and (l) Absolute count of CNS Foxp3+ CD4+, and IL-17+ CD4+ T cells, respectively in the EAE mice measured by FACS 12 days after immunization. Data are expressed as the means ± SEM; **P < or = 0.007, Student’s t test. (m) Absolute count of CNS CD19+ cells following treatment with vehicle or MDP in EAE mice as measured by FACS 12 days after immunization. (n) Immunoblot analysis of Iba1 protein expression in the CNS showed no significant difference between groups