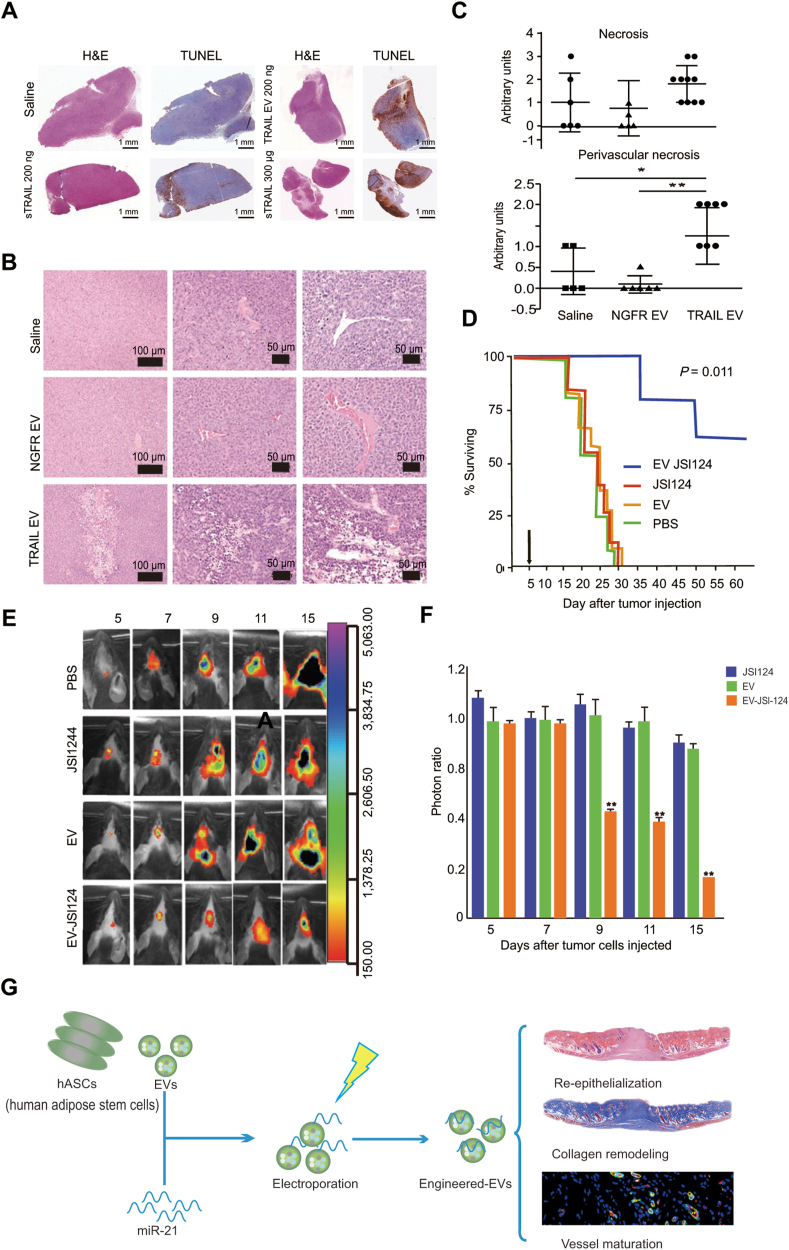
Figure 8.
Applications of extracellular vesicles in drug delivery. (A)‒(C) The antitumor activity of TRAIL extracellular vesicle treatments against KMS11 multiple myeloma. (A) Intratumor treatment. KMS11-bearing mice were treated by TRAIL EVs every 48 h and then measured by the TUNEL assay. (B) and (C) Systematic treatment. Hematoxylin and eosin staining images (B) and determination of necrotic areas (C) of tumors of KMS11-bearing mice were treated by four intravenous treatments of TRAIL EVs, NGFR EVs, or saline every 48 h (n = 5–6 saline and mice accepting NGFR EVs; n = 10 mice accepting TRAIL EVs). Adapted with permission from Ref. 195. Copyright © 2016 American Association for Cancer Research. (D)–(F) Treatment with JSI124-EVs prevents the in vivo growth of injected brain tumor cells. (D) The surviving rate of mice treated with EV-JSI124, JSI124, EV and control administration. (E) and (F) A representative photo of brain tumor signals (E) and the growth potential of cells (F) of mice treated with JSI124, EV, or EV-JSI124. Adapted with permission from Ref. 190. Copyright © 2011 The American Society of Gene & Cell Therapy. (G) The human adipose stem cell-derived EVs loaded with miR-21-5p improved diabetic wound healing. Adapted with permission from Ref. 194. Copyright © 2020 American Chemical Society.