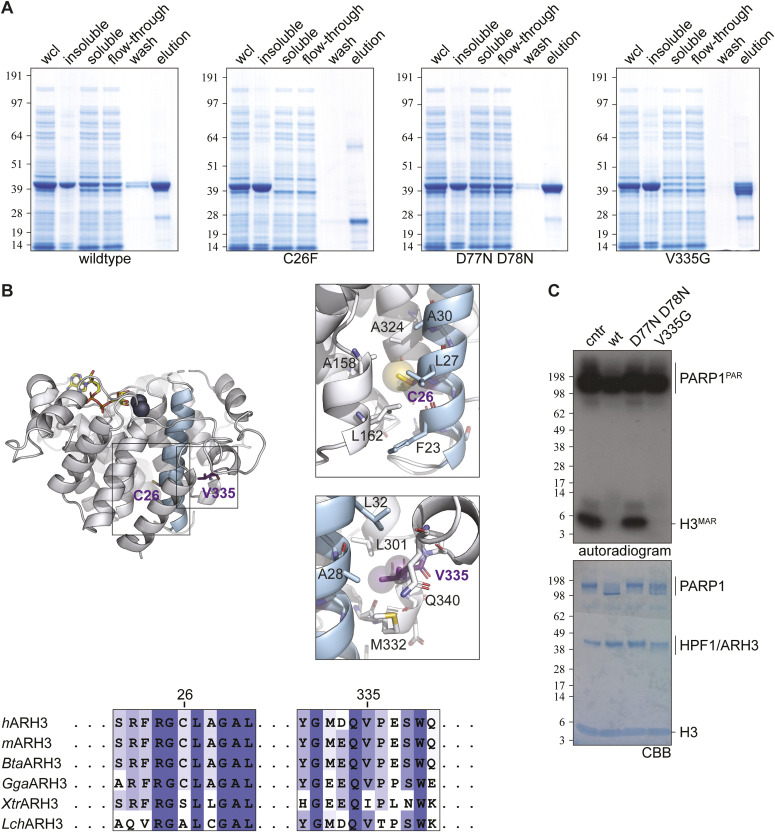
Figure 2. In vitro expression and activity of ARH3 (mutant) protein and ribbon representation of ARH3 in complex with ADP ribosylation (yellow) and Mg2+ ions (dark blue).
(A) SDS–PAGE analysis of expression and purification of recombinant ARH3 wild type and mutants in Escherichia coli. ARH3 (theoretical Mw 42.88 kD) was enrich from whole cell lysate by nickel affinity chromatography (for details, see the Materials and Methods section). Both C26F and V335G show similar expression, but lower abundance in the soluble fraction, compared with WT and D77N D778N mutant. (B) Alpha-helix 1, containing Cys26, is highlighted for orientation purposes. Right panels: Van der Waals radii of Cys26 sulphur and Val335 side chain carbon atoms are depicted as transparent spheres. Residue Cys26 is located in the core of a conserved helical bundle (right upper panel). Positioning of this residue within the structure suggest that the increase in Van der Waals volume associated with the C26F mutation incompatible with correct packing. Residue Val335 is located in partial structured surface loop packing against α-helix 1 (right lower panel) and is inserted in a hydrophobic pocket. The structural consequences of the V335G mutation are not immediately appreciable but may weaken the local packing, expose hydrophobic residues and thus affect the overall structural stability of the protein. Note that in the right panels foreground structural elements have been removed to allow representation of the buried residue pockets. Image was created with PyMOL v2.3 (Schrodinger LLC) using human ARH3 in complex with ADP-ribose (PDB 6D36). (C) The (ADP-ribosyl)hydrolase activity of ARH3 WT and mutants was assessed using H3 and poly(ADP-ribose)polymerase (PARP)1 MARylated and PARylated, respectively, in presence of 32P-NAD+ as substrates. After the reaction samples were analyzed by autoradiogram and SDS–PAGE. Both WT and V335G were active under the assay conditions. cntr (control; no ARH3).