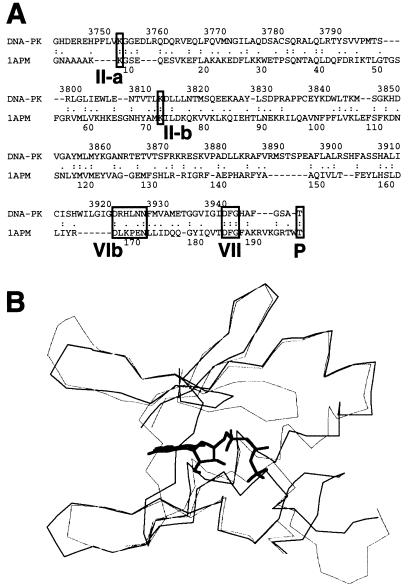
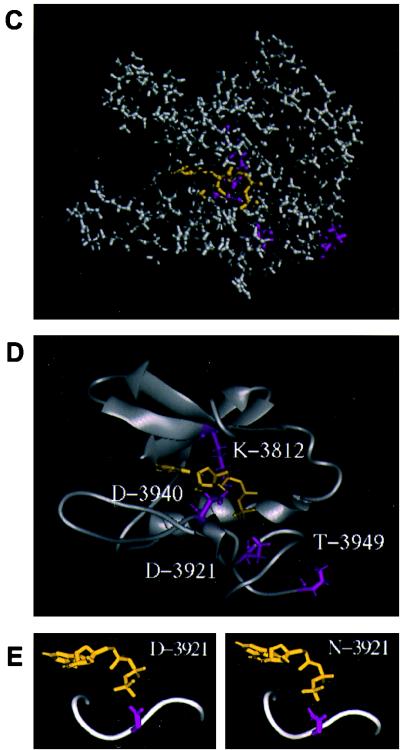
FIG. 5.
Three-dimensional modeling of the DNA-PKcs PI 3-kinase domain. (A) The sequence of the ATP binding pocket of the human DNA-PKcs was aligned with that of the catalytic subunit of cAPK (1APM). Each open box shows the serine/threonine protein kinase subdomain II homology domain within the PI 3-kinase superfamily (II-a), subdomain II from 1APM (II-b), and subdomain VIb and VII homology regions within DNA-PKcs and 1APM (VIb, VII). The phosphorylation site of threonine near the catalytic site is also shown as an open box (P). (B) Superimposed image of the ATP binding pocket of the modeled DNA-PKcs structure and the crystal structure of the 1APM active site. The thick black line in the center is ANP, the thin black line is the modeled structure of the DNA-PKcs, and the thin gray line is the crystal structure of 1APM. (C) Energy-minimized structure of the ATP binding pocket of the human DNA-PKcs. The ATP binding pocket is drawn in light gray, with ANP drawn in yellow and three conserved residues (K-3812, D-3921, and D-3940) drawn in magenta. (D) The backbone fold image of the binding pocket of the DNA-PKcs is shown together with ATP, the two conserved residues (K-3812 and D-3940) that are important for ATP binding, and another conserved residue, D-3921, that contributes to catalytic activity. The glycosylated T-3949, which may contribute to control kinase activity by phosphorylation, is also shown. (E) Structure model of the mutated residue D3921N of kinase subdomain VIb. The catalytic aspartate D-3921 is oriented with the carboxyl group facing the γ-phosphate of ATP (left). In the mutant molecule, N-3921 has an amino group pointing toward the γ-phosphate of ATP (right).