Abstract
Vertical sleeve gastrectomy (VSG) is a surgical weight loss procedure that resects 80% of the stomach, creating a tube linking the esophagus to the duodenum. Because of the efficacy and relative simplicity of VSG, it is preferred in the United States, with VSG currently at >61% of bariatric surgeries performed. Surprisingly, there has never been a complete molecular characterization of the human stomach greater curvature’s fundus and corpus. Here we compare and contrast the molecular makeup of these regions. We performed a prospective cohort study to obtain gastric tissue samples from patients undergoing elective VSG. Paired fundus and corpus samples were obtained. Whole genome transcriptome analysis was performed by RNA sequencing (N = 10), with key findings validated by qPCR (N = 24). Participants were primarily female (95.8%) and White (79.15%). Mean body mass index, body weight, and age were 46.1 kg/m2, 121.6 kg, and 43.29 yr, respectively. Overall, 432 gene transcripts were significantly different between the fundus and the corpus (P < 0.05). A significant correlation was found between the RNA sequencing dataset and qPCR validation, demonstrating robust gene expression differences between the fundus and the corpus. Significant genes included progastricsin, acidic chitinase, and gastokine 1 and 2 in both the fundus and the corpus. Of the very highly expressed genes in both regions, 87% were present in both the stomach’s fundus and corpus, indicating substantial overlap. Despite significant overlap in the greater curvature gene signature, regional differences exist within the fundus and the corpus. Given that the mechanism of VSG is partly unresolved, the potential that the resected tissue may express genes that influence long-term body weight regulation is unknown and could influence VSG outcomes.
Keywords: bariatric surgery, diabetes, fundus, greater curvature, obesity, stomach
INTRODUCTION
Surgical weight loss is the most successful method to reduce the burden of excess body weight, type 2 diabetes mellitus (T2DM), dyslipidemia, and hypertension (1, 2). Although more than 250,000 individuals obtain surgical weight loss procedures per year in the United States alone (3), the underpinnings of the mechanisms of action to produce positive benefits remain unresolved. The mechanism is far more complicated than merely limiting the stomach’s volume and concomitantly reducing food intake.
Vertical sleeve gastrectomy (VSG) is a surgical weight loss procedure that resects greater than 80% of the stomach, creating a tube linking the esophagus to the duodenum. VSG reduces the volume of the stomach and produces uninhibited gastric emptying (4), which has a downstream impact on the adjoining intestine. As a result, patients who obtain VSG can expect a significant excess loss of body weight, remission of T2DM, and reduced needs for hypertensive medications. Because of this surgery’s great effectiveness and relative simplicity, VSG is now on the rise in the United States, comprising over 61% of the total bariatric surgeries performed (3). The gold standard for the resolution of obesity comorbidities remains Roux-en-Y gastric bypass (RYGB). In many ways, VSG produces similar weight loss benefits to RYGB despite the significant differences in the surgical procedure (5, 6). In the case of RYGB, nutrients are excluded from contact with the fundus and the corpus through fashioning of the gastric pouch, in addition to manipulation of the hindgut. VSG is unique in that a great percentage of both the fundus and the corpus of the stomach is altogether resected from the body. Nonetheless, manipulation of the greater curvature of the stomach is common in both procedures. It stands to reason that the greater curvature’s molecular constituents may influence the improvement of metabolic health, either through resection or by increasing the relative influence of the remaining lesser curvature gastric tissue on gastrointestinal physiology.
Given the significant tissue resection in VSG, it would be expected that a complete molecular characterization of the fundus and corpus gastric tissue would have been previously investigated in humans. However, to our knowledge, no such report has been identified in the literature. Thus, to address this gap in knowledge, gastric tissue was collected from study participants obtaining elective VSG at the University of Mississippi Medical Center in Jackson (UMMC), MS. Transcriptome analysis using RNA sequencing (RNAseq) was performed on a subset of paired tissue biopsies (N = 10) obtained from the resected fundus and corpus. Subsequently, using paired gastric samples (N = 24), the identified differential expression was validated by real-time PCR.
METHODS
Ethics Approval
All ethics approval and consent to participate were obtained under UMMC IRB 2014-0047.
Assurances
All procedures were performed in accordance with the 1964 Declaration of Helsinki ethical standards. Written informed consent was obtained from each participant before formally entering this Institutional Review Board (IRB)-approved study, Predictors of Weight Loss (POWL), at the University of Mississippi Medical Center (UMMC), Jackson, MS (IRB No. 2014-0047).
Participants
Patients receiving elective VSG surgery through the UMMC Weight Management Clinic were consented. This study’s inclusion criteria comprised men and women between the ages of 21 and 65 yr, body mass index (BMI) of ≥35 kg/m2, and underwent first-time bariatric procedures between June and December 2016. Exclusion criteria for participation in this study were as follows: 1) psychiatric conditions such as current substance abuse or dependence, mania, psychosis, or dementia or the inability to give informed consent; 2) history of major organ system failure such as end-stage liver disease, severe renal insufficiency (estimated glomerular filtration rate < 30 mL/min/1.73 m2) or dialysis, and severe arterial insufficiency as defined by moderate-to-severe claudication; 3) use of medications that affect body weight or metabolic rate, including antipsychotic drugs (clozapine, olanzapine, paliperidone, and quetiapine), β-adrenergic blocking drugs (carvedilol, labetalol, nadolol, propranolol, atenolol, or metoprolol), and anorectic drugs (phentermine, topiramate, lorcaserin, exenatide, or liraglutide); 4) history of cancer within the past 5 years; 5) solid-organ transplant recipients; 6) pregnant or lactating women; and 7) prior recipients of RYGB, VSG, gastric banding, or biliopancreatic diversion.
Tissue Collection
The fundus and the corpus of the greater curvature were collected immediately after the removal of the tissue during surgery. Due to the great variability of the size of the resected stomach (due to some patients having markedly stretched stomachs), the fundus and corpus samples were obtained from either pole of the sample removed during surgery. The fundus and corpus samples were separated by a distance of at least 10 cm. Samples were either flash frozen and carried to the research laboratory where they were stored at −80°C until sample extraction.
Isolation of RNA
Gastric RNA was isolated with TRIzol and extracted using a QIAGEN miniprep RNA kit (No. 74104, QIAGEN, Inc, Valencia, CA). RNA content was quantified using the NanoDrop Lite (Fisher ThermoScientific, Waltham, MA). All samples displayed a purity level of greater than 1.8 as measured by OD260/280 ratio.
RNA Sequencing
RNA was assessed for quality control parameters of minimum concentration and fidelity (i.e., 18S and 28S bands, RNA quality indicator (RQI) >8). Libraries were developed using the TruSeq mRNA Stranded Library Prep Kit (Set-A-indexes), quantified with the Qubit fluorimeter (Invitrogen), and assessed for quality and size using the Bio-Rad Experion System. Samples were pooled into a single library (n = 10 pooled samples per library) and sequenced using the NextSeq 500 High Output Kit (300 cycles, paired-end 100 bp) on the Illumina NextSeq 500 platform. The run generated 124 Gb at QC30 = 85.6% with 605 million or 60 million reads per sample passing filter. Sequenced reads were assessed for quality using the Illumina BaseSpace Cloud Computing Platform, and FASTQ sequence files were used to align reads to the human reference genome [Homo sapiens/hg19 (RefSeq)] using the RNA-Seq Alignment Application (using STAR aligner). Differential expression was determined using the Cufflinks Assembly & DE workflow (v2.1.0) or DESeq2. Gene expression differences are denoted as log2 (ratio) and q > 0.05. We used a rational approach to the validation of the transcripts presented here. We first identified the most abundant transcripts in both the fundus and the corpus. Then, we performed a literature review of the genes that were most highly expressed with an eye to identify genes that have been reported to have a physiological role that may influence bariatric outcomes. We then followed up with validation of said transcripts of interest.
TaqMan Real-Time PCR
Total RNA was reverse transcribed and converted to cDNA (complementary DNA) using an iScript cDNA synthesis kit (No. 1708840, Bio-Rad Laboratories, Hercules, CA). Quantitative polymerase chain reaction (qPCR) was performed on a Step-One Plus Real-Time PCR machine coupled with StepOne Software (v2.3) (Applied Biosystems) using TaqMan inventoried gene expression assays (Life Technologies, Foster City, CA). Samples were analyzed in duplicate, and changes in threshold cycle (Ct) values from the internal control 60 s ribosomal protein 32 (RPL32) were calculated. The control group average ΔCt was made to equal 1. ΔCts of the control group and the experimental groups were then compared, and the fold change was calculated, creating a 2ΔΔCt paradigm. Data were then multiplied by 100 so that the control group average ΔCt equaled 100.
Statistics
All statistical analyses were performed using GraphPad Prism version 8.1.2 (GraphPad Software, San Diego, CA). Statistical significance was determined with a paired Student’s t test. All results are given as means ± SE. Results were considered statistically significant when P < 0.05.
RESULTS
Participant Characteristics
Consecutively consented individuals for VSG were predominantly female (95.8%) and White (79.15%) (Table 1). The average age of the participants was 43.3 yr. The average BMI was 46.1 kg/m2, and the average body weight was 121.6 kg (Table 1). Metabolic comorbidities that accompanied obesity in this group included obstructive sleep apnea (25%), hypertension (54.1%), hyperlipidemia (20.8%), and T2DM (20.8%) (Table 1). Other laboratory values for this obese cohort are included in Table 1.
Table 1.
Participant characteristics
| Participant Characteristics | Baseline | SE | Range |
|---|---|---|---|
| Females/Total | 23/24 | N/A | |
| White/Total | 19/24 | N/A | |
| Black/Total | 5/24 | N/A | |
| Age, yr | 43.29 | ±2.25 | 20.6–63.75 |
| BMI, kg/m2 | 46.1 | ±1.27 | 37.06–45.86 |
| Body weight, kg | 121.6 | ±3.92 | 97.98–179.6 |
| Waist circumference, cm | 50.0 | ±1.36 | 41–68.75 |
| Hip circumference, cm | 53.4 | ±1.19 | 38.25–66 |
| Fasting LDL cholesterol, mg/dL | 100.8 | ±6.03 | 49–157 |
| Fasting HDL cholesterol, mg/dL | 49.1 | ±2.61 | 29–74 |
| Fasting triglyceride, mg/dL | 113.7 | ±8.73 | 56–208 |
| Fasting plasma glucose, mg/dL | 105.2 | ±4.18 | 70–158 |
| ALT, IU/L | 29.5 | ±3.22 | 5–69 |
| AST, IU/L | 26.5 | ±2.21 | 11–54 |
| Bilirubin, µmol/L | 0.5 | ±0.05 | 0.2–1.1 |
| Creatinine | 0.7 | ±0.03 | 0.4–1.07 |
| Systolic blood pressure, mmHg | 127.8 | ±3.00 | 104–155 |
| Diastolic blood pressure, mmHg | 77.9 | ±2.25 | 56–101 |
| Pulse, beats/min | 86.4 | ±3.15 | 64–121 |
| Obstructive sleep apnea, Y/N | 6/24 | N/A | |
| Hypertension, Y/N | 13/24 | N/A | |
| Hyperlipidemia, Y/N | 5/24 | N/A | |
| Diabetes, Y/N | 5/24 | N/A |
Values are reported as total numbers or means ± SE and range of values. n = 24 participants. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; N/A; not applicable; Y/N, yes/no.
Whole Transcriptome Analysis
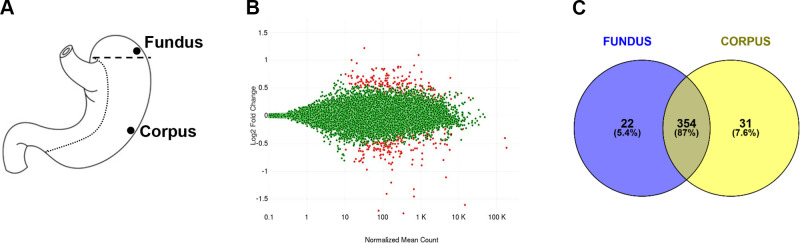
Two general regions (fundus and corpus) of the stomach’s greater curvature were compared (Fig. 1A). On average, the fundus had 43 million reads per sample (or >95% reads) mapped to the reference genome. The corpus had, on average, 41 million reads per sample (or >95% reads) mapped to the reference genome. Differential expression was determined using DESeq2 (v1.1.0). There were 432 gene transcripts found to be significantly different between the fundus and the corpus (P < 0.05) (Fig. 1B). We arbitrarily set a high-threshold expression level of 175 for FPKM (fragments per kilobase of exon model per million reads mapped) values. We next compared the FPKM for each region, which amounted to 376 genes in the fundus and 385 genes in the corpus. Of these highly expressed genes, 87% of the genes were present in both the fundus and the corpus (Fig. 1C), indicating a vast overlap between these two areas of the stomach.
Figure 1.
A: diagram of the human stomach with general location of fundus and corpus samples. B: MA plot generated by differential gene expression analysis of the RNA sequencing (RNAseq) dataset. Differential gene expression is visualized with the fold change (log2) on the y-axis over the mean of normalized counts. The red dots represent genes with significantly different expression (P < 0.05). C: Venn diagram representing the percent of significantly different gene expression in the fundus or corpus, along with overlapping expression in both regions.
The Most Highly Enriched Genes in the Fundus versus Corpus
The top 25 highest FPKM values for the fundus with corresponding corpus FPKM values are listed in chronological order in Table 2. The highest FPKM values identified in the corpus were similar to those for the fundus: progastricsin (PGC), gastokine 1 (GKN1), RNA component processing endoribonuclease (RMRP), trefoil factor 1 (TFF1), and microRNA 6087 (MIR6087) (Table 2). The top 25 highest FPKM values for the corpus with corresponding fundus FPKM values are also listed in chronological order in Table 2. In the corpus, the highest FPKM values identified were similar to those for the fundus: PGC, GKN1, RMRP, TFF1, and microRNA 6087 (MIR6087). The FPKM average values for genes in the fundus were not necessarily statistically different than FPKM values in the corpus. Of this short list, only PGC, acidic chitinase (CHIA1), serine peptidase inhibitor 1 (SPINK1), ghrelin (GHRL), and β-actin (ACTB) are differentially expressed as delineated by P value (shaded in gray) (Table 2).
Table 2.
RNAseq data of genes with the highest mean expression in the gastric fundus and corpus
| Symbol | Gene Name | Fundus (Means ± SE) | Corpus (Means ± SE) | log2 (FC) | P Value |
|---|---|---|---|---|---|
| Highest expression in the fundus | |||||
| PGC | Progastricsin | 94,228.9 ± 0.0 | 40,028.3 ± 4,012.7 | 1.24 | 0.00 |
| GKN1 | Gastrokine 1 | 29,178.2 ± 5,208.1 | 31,363.0 ± 7,255.5 | −0.10 | 0.57 |
| RMRP | RNA component processing endoribonuclease | 10,001.7 ± 5,083.1 | 12,166.5 ± 6,285.0 | −0.28 | 0.81 |
| TFF1 | Trefoil factor 1 | 6,123.2 ± 890.5 | 8,081.4 ± 1,592.0 | −0.40 | 0.24 |
| TMSB4X | Thymosin β 4 X-linked | 4,342.2 ± 647.2 | 4,209.9 ± 461.6 | 0.04 | 0.84 |
| MT1G | Metallothionein 1 G | 3,801.2 ± 1,008.7 | 2,034.9 ± 545.6 | 0.90 | 0.15 |
| MIR6087 | MicroRNA 6087 | 3,556.4 ± 1,820.1 | 6,064.6 ± 4,489.4 | −0.77 | 0.63 |
| PSCA | Prostate stem cell antigen | 3,327.2 ± 932.7 | 3,471.7 ± 623.2 | −0.06 | 0.92 |
| REG1A | Regenerating family member 1 α | 3,118.5 ± 818.0 | 1,953.7 ± 603.3 | 0.67 | 0.25 |
| TFF2 | Trefoil factor 2 | 3,047.6 ± 511.8 | 3,380.2 ± 522.1 | −0.15 | 0.61 |
| GIF | Gastric intrinsic factor | 2,636.0 ± 246.8 | 2,605.5 ± 245.2 | 0.02 | 0.93 |
| B2M | β-2-Microglobulin | 2,608.8 ± 325.5 | 2,280.7 ± 252.6 | 0.19 | 0.47 |
| GKN2 | Gastrokine 2 | 2,398.2 ± 351.1 | 3,122.8 ± 693.0 | −0.38 | 0.33 |
| FTL | Ferritin light chain | 2,224.6 ± 196.0 | 1,906.7 ± 190.2 | 0.22 | 0.24 |
| MT2A | Metallothionein 2 A | 2,009.5 ± 466.5 | 1,259.0 ± 321.6 | 0.67 | 0.17 |
| CHIA | Chitinase, acidic | 1,788.7 ± 284.5 | 355.8 ± 134.5 | 2.33 | 0.00 |
| EEF1A1 | Eukaryotic translation elongation factor 1 α 1 | 1,596.3 ± 83.1 | 1,498.3 ± 103.5 | 0.09 | 0.50 |
| FTH1 | Ferritin heavy chain 1 | 1,546.2 ± 190.9 | 1,550.1 ± 179.9 | 0.00 | 0.99 |
| SPINK1 | Serine peptidase inhibitor, Kazal type 1 | 1,459.2 ± 259.4 | 742.5 ± 162.9 | 0.97 | 0.03 |
| JCHAIN | Joining chain of multimeric IgA and IgM | 1,441.8 ± 491.3 | 798.7 ± 130.5 | 0.85 | 0.30 |
| CYSTM1 | Cysteine-rich transmembrane module containing 1 | 1,378.2 ± 124.3 | 1,320.8 ± 74.1 | 0.06 | 0.73 |
| S100P | S100 calcium-binding protein P | 1,342.6 ± 207.3 | 1,313.7 ± 220.7 | 0.03 | 0.92 |
| ATP4B | ATPase H+/K+ transporting subunit β | 1,287.0 ± 154.0 | 1,506.3 ± 235.5 | −0.23 | 0.51 |
| GHRL | Ghrelin and obestatin prepropeptide | 1,221.1 ± 132.2 | 417.2 ± 62.1 | 1.55 | 0.00 |
| MYL6 | Myosin light chain 6 | 1,197.5 ± 201.6 | 2,052.3 ± 450.7 | −0.78 | 0.15 |
| Highest expression in the corpus | |||||
| PGC | Progastricsin | 94,228.90 ± 0.00 | 40,028.25 ± 4,012.66 | 1.24 | 0.00 |
| GKN1 | Gastrokine 1 | 29,178.20 ± 5,208.14 | 31,362.96 ± 7,255.46 | −0.10 | 0.57 |
| RMRP | RNA component of endoribonuclease | 10,001.69 ± 5,083.09 | 12,166.53 ± 6,285.04 | −0.28 | 0.81 |
| TFF1 | Trefoil factor 1 | 6,123.25 ± 890.46 | 8,081.36 ± 1,591.97 | −0.40 | 0.24 |
| MIR6087 | MicroRNA 6087 | 3,556.43 ± 1,820.11 | 6,064.64 ± 4,489.41 | −0.77 | 0.63 |
| TMSB4X | Thymosin β 4 X-linked | 4,342.22 ± 647.22 | 4,209.93 ± 461.59 | 0.04 | 0.84 |
| PSCA | Prostate stem cell antigen | 3,327.16 ± 932.69 | 3,471.67 ± 623.19 | −0.06 | 0.92 |
| TFF2 | Trefoil factor 2 | 3,047.58 ± 511.83 | 3,380.24 ± 522.09 | −0.15 | 0.61 |
| GKN2 | Gastrokine 2 | 2,398.22 ± 351.14 | 3,122.76 ± 693.04 | −0.38 | 0.33 |
| GIF | Gastric intrinsic factor | 2,636.04 ± 246.80 | 2,605.50 ± 245.21 | 0.02 | 0.93 |
| B2M | β-2-Microglobulin | 2,608.83 ± 325.51 | 2,280.68 ± 252.64 | 0.19 | 0.47 |
| MYL6 | Myosin light chain 6 | 1,197.55 ± 201.64 | 2,052.26 ± 450.67 | −0.78 | 0.15 |
| MT1G | Metallothionein 1 G | 3,801.20 ± 1,008.68 | 2,034.94 ± 545.63 | 0.90 | 0.15 |
| REG1A | Regenerating family member 1 α | 3,118.52 ± 818.05 | 1,953.73 ± 603.26 | 0.67 | 0.25 |
| FTL | Ferritin light chain | 2,224.62 ± 195.97 | 1,906.73 ± 190.21 | 0.22 | 0.24 |
| TAGLN | Transgelin | 795.11 ± 279.53 | 1,839.48 ± 545.81 | −1.21 | 0.18 |
| FTH1 | Ferritin heavy chain 1 | 1,546.17 ± 190.93 | 1,550.11 ± 179.90 | 0.00 | 0.99 |
| ATP4B | ATPase H+/K+ transporting subunit β | 1,286.95 ± 153.96 | 1,506.30 ± 235.52 | −0.23 | 0.51 |
| EEF1A1 | Eukaryotic translation elongation factor 1 α 1 | 1,596.29 ± 83.07 | 1,498.32 ± 103.54 | 0.09 | 0.50 |
| ACTB | Actin β | 957.18 ± 47.84 | 1,421.54 ± 118.41 | −0.57 | 0.02 |
| ACTG2 | Actin, γ 2, smooth muscle, enteric | 319.22 ± 141.73 | 1,362.77 ± 458.73 | −2.09 | 0.08 |
| ATP4A | ATPase H+/K+ transporting subunit α | 1,179.33 ± 108.06 | 1,325.53 ± 139.09 | −0.17 | 0.47 |
| CYSTM1 | Cysteine-rich transmembrane module containing 1 | 1,378.24 ± 124.27 | 1,320.76 ± 74.07 | 0.06 | 0.73 |
| S100P | S100 calcium-binding protein P | 1,342.64 ± 207.34 | 1,313.68 ± 220.68 | 0.03 | 0.92 |
| MT2A | Metallothionein 2 A | 2,009.46 ± 466.53 | 1,258.98 ± 321.56 | 0.67 | 0.17 |
| MYL9 | Myosin light chain 9 | 412.15 ± 161.28 | 1,257.32 ± 394.02 | −1.61 | 0.11 |
Genes were sorted and selected using mean expression in the corpus ± SE. Genes that are bolded were among those validated by qPCR. FC, fold change.
We compared the FPKM values of the fundus and corpus to determine differential expression between the two gastric regions. Differences between fundus and corpus are listed by fold change and respective q value (Table 3).
Table 3.
RNAseq data of genes with the highest amount of overlapping expression in both regions of the stomach sorted by fold change
| Gene | Gene Name | log2 (Fundus) | log2 (Corpus) | log2 (FC) | q Value |
|---|---|---|---|---|---|
| TTR | Transthyretin | 9.07 | 7.31 | −1.76 | 0.00E + 00 |
| CHIAP2 | Chitinase, acidic pseudogene 2 | 6.88 | 5.17 | −1.71 | 0.00E + 00 |
| CHIA | Chitinase, acidic | 14.41 | 12.81 | −1.61 | 0.00E + 00 |
| SIM2 | Single-minded family BHLH transcription factor 2 | 9.91 | 8.46 | −1.45 | 0.00E + 00 |
| IRX5 | Iroquois homeobox 5 | 7.51 | 6.1 | −1.41 | 0.00E + 00 |
| GC | GC, vitamin D-binding protein | 9.87 | 8.54 | −1.32 | 0.00E + 00 |
| MSLN | Mesothelin | 7.85 | 6.65 | −1.2 | 0.00E + 00 |
| GHRL | Ghrelin and obestatin prepropeptide | 12.62 | 11.51 | −1.11 | 0.00E + 00 |
| TM4SF4 | Transmembrane 4 L six family member 4 | 7.86 | 6.89 | −0.97 | 0.00E + 00 |
| EYA1 | EYA transcriptional coactivator and phosphatase 1 | 5.65 | 4.67 | −0.97 | 2.00E-06 |
| C8orf4 | Chromosome 8 open reading frame 4 | 10.42 | 9.52 | −0.89 | 0.00E + 00 |
| UGT2B15 | UDP glucuronosyltransferase family 2 member B15 | 9.57 | 8.69 | −0.88 | 1.00E-06 |
| PKHD1 | PKHD1, fibrocystin/polyductin | 7.73 | 6.85 | −0.87 | 0.00E + 00 |
| IYD | Iodotyrosine deiodinase | 7.54 | 6.69 | −0.86 | 8.00E-06 |
| FAR2P2 | Fatty acyl-CoA reductase 2 pseudogene 2 | 5.24 | 4.4 | −0.84 | 1.90E-05 |
| ALB | Albumin | 7.83 | 6.99 | −0.84 | 2.80E-04 |
| CRYBA2 | Crystallin β A2 | 4.51 | 3.68 | −0.83 | 3.56E-04 |
| CLDN4 | Claudin 4 | 4.92 | 4.1 | −0.82 | 1.84E-04 |
| VTN | Vitronectin | 5.04 | 4.23 | −0.81 | 4.32E-04 |
| CHGA | Chromogranin A | 12.29 | 11.49 | −0.8 | 4.00E-06 |
| NUPR1L | Nuclear protein, transcriptional regulator, 1-like | 7.18 | 6.4 | −0.79 | 2.00E-06 |
| SERPINA1 | Serpin family A member 1 | 9.59 | 8.8 | −0.79 | 1.24E-04 |
| SST | Somatostatin | 10.42 | 9.64 | −0.78 | 4.32E-04 |
| LGR5 | Leucine-rich repeat-containing G protein-coupled receptor 5 | 5.12 | 4.35 | −0.78 | 9.30E-04 |
| SLC30A8 | Solute carrier family 30 member 8 | 4.62 | 3.84 | −0.78 | 1.09E-03 |
| BHMT | Betaine—homocysteine S-methyltransferase | 6.8 | 6.03 | −0.76 | 0.00E + 00 |
| TNS4 | Tensin 4 | 5.3 | 4.54 | −0.76 | 9.70E-05 |
| EYA2 | EYA transcriptional coactivator and phosphatase 2 | 7.88 | 7.13 | −0.75 | 5.60E-05 |
| TAC1 | Tachykinin precursor 1 | 2.96 | 3.7 | 0.75 | 5.88E-04 |
| MMD | Monocyte to macrophage differentiation associated | 6.56 | 7.31 | 0.76 | 7.90E-05 |
| FABP4 | Fatty acid-binding protein 4 | 8.56 | 9.34 | 0.78 | 3.56E-04 |
| GATA4 | GATA-binding protein 4 | 9.59 | 10.38 | 0.79 | 4.00E-06 |
| THRSP | Thyroid hormone responsive | 3.53 | 4.33 | 0.8 | 4.32E-04 |
| SAA1 | Serum amyloid A1 | 7.17 | 7.98 | 0.81 | 1.70E-05 |
| GUCA2B | Guanylate cyclase activator 2B | 7.15 | 7.96 | 0.81 | 1.45E-04 |
| SIX2 | SIX homeobox 2 | 6.23 | 7.05 | 0.82 | 3.52E-04 |
| TAC3 | Tachykinin 3 | 5.35 | 6.16 | 0.82 | 4.68E-04 |
| ADIPOQ | Adiponectin, C1Q and collagen domain containing | 7.09 | 7.91 | 0.83 | 4.17E-04 |
| CIDEC | Cell death-inducing DFFA-like effector C | 6.08 | 6.92 | 0.84 | 1.19E-04 |
| TUSC5 | Tumor suppressor candidate 5 | 5.21 | 6.05 | 0.84 | 2.71E-04 |
| CPB1 | Carboxypeptidase B1 | 6.29 | 7.13 | 0.84 | 2.82E-04 |
| TNFRSF12A | TNF receptor superfamily member 12 A | 5.57 | 6.42 | 0.85 | 5.60E-05 |
| PLIN1 | Perilipin 1 | 7.47 | 8.33 | 0.86 | 1.08E-04 |
| MUC13 | Mucin 13, cell surface associated | 7.68 | 8.56 | 0.87 | 1.04E-04 |
| MTCL1 | Microtubule cross-linking factor 1 | 7.47 | 8.35 | 0.88 | 6.00E-06 |
| CA12 | Carbonic anhydrase 12 | 8.85 | 9.75 | 0.9 | 6.70E-05 |
| POU6F2-AS1 | POU6F2 antisense RNA 1 | 3.81 | 4.75 | 0.94 | 1.90E-05 |
| CIDEA | Cell death-inducing DFFA-like effector A | 3.32 | 4.31 | 1 | 4.00E-06 |
| AQP4 | Aquaporin 4 | 9.12 | 10.21 | 1.09 | 0.00E + 00 |
| LINC00520 | Long intergenic nonprotein coding RNA 520 | 4.29 | 5.5 | 1.21 | 0.00E + 00 |
A cutoff value of ≥ 0.75 (FC) was used to select these genes. Genes that are bolded were among those validated by qPCR. FC, fold change.
Genes Enriched in the Fundus
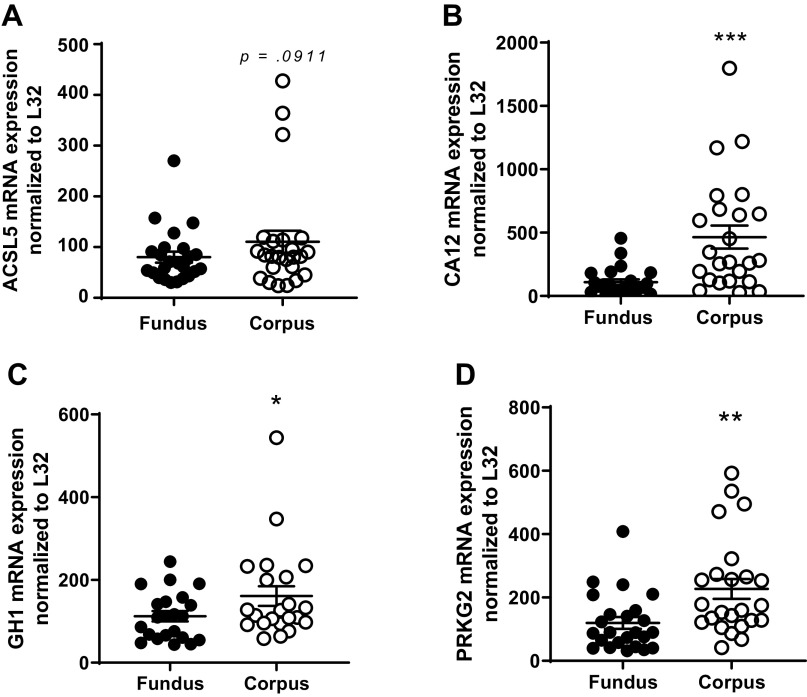
Based on the RNAseq dataset, genes significantly elevated in the fundus compared with in the corpus were selected for qPCR validation. The genes include CHIA1, P < 0.001 (Fig. 2A); vitamin D-binding protein (GC), P < 0.01 (Fig. 2B); GHRL, P < 0.001 (Fig. 2C); iroquois homeobox 5 (IRX5), P < 0.01 (Fig. 2D); mesothelin (MSLN), P < 0.01 (Fig. 2E); PGC, P < 0.01 (Fig. 2F); regenerating family member 1-α (REG1A), P < 0.05 (Fig. 2G); somatostatin (SST), P < 0.05 (Fig. 2H); and transthyretin (TTR), P < 0.001 (Fig. 2I).
Figure 2.
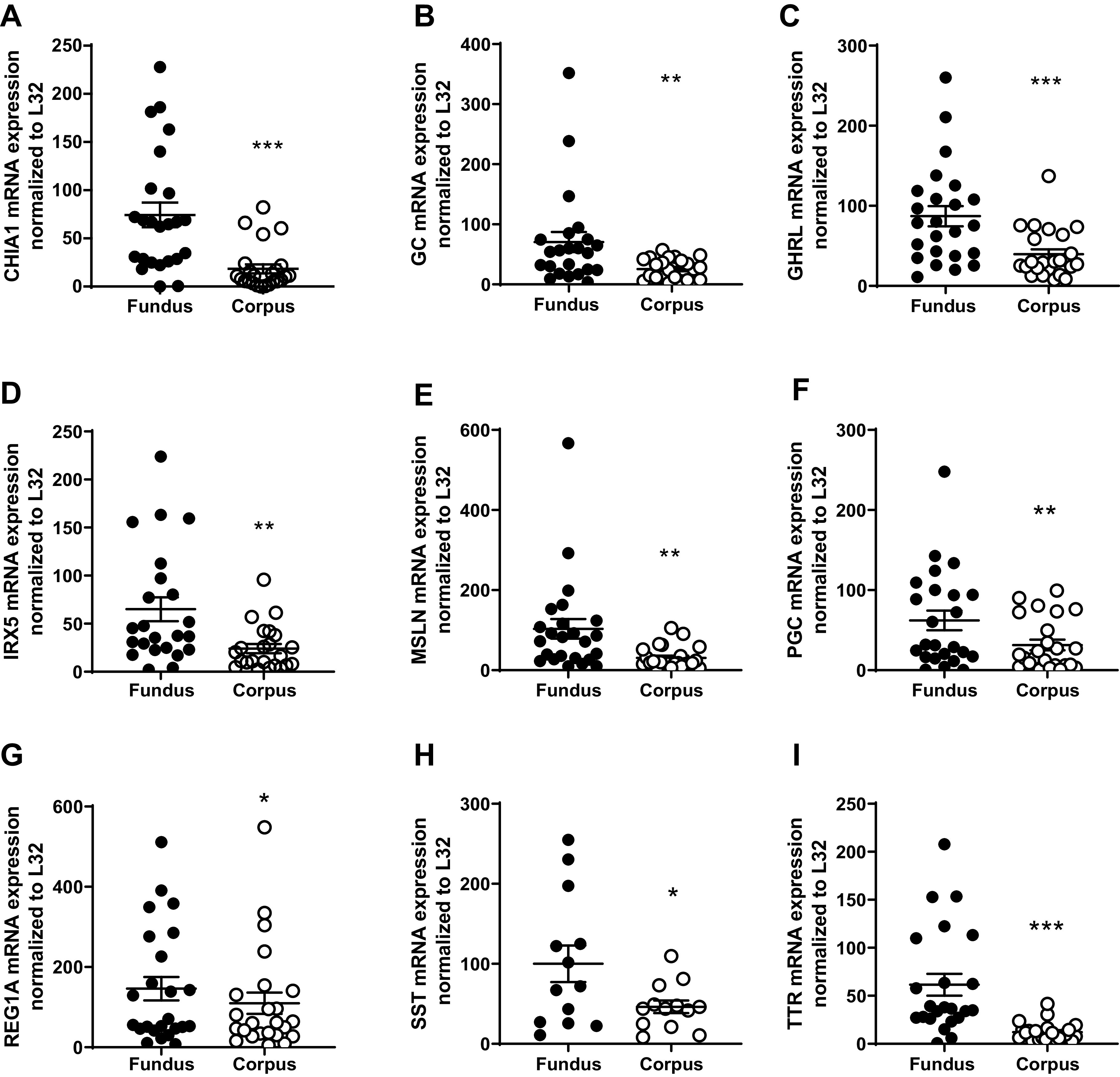
qPCR validation of the RNA sequencing (RNAseq) of upregulated genes highly expressed in the fundus versus the corpus. A: acidic chitinase (CHIA1); B: vitamin D-binding protein (GC); C: ghrelin (GHRL); D: iroquois homeobox 5 (IRX5); E: mesothelin (MSLN); F: progastricsin (PGC); G: regenerating family member 1-α (REG1A); H: somatostatin (SST); and I: transthyretin (TTR). Data are presented as means ±SE. n = 24/region, *P < 0.05, **P < 0.01, ***P < 0.001.
Genes Enriched in the Corpus
Based on the RNAseq dataset, genes elevated in the corpus compared with in the fundus were identified and validated, including acyl-CoA synthetase long-chain family member 5 (ACSL5), P = 0.0911 (Fig. 3A); carbonic anhydrase 12 (CA12), P < 0.001 (Fig. 3B); growth hormone 1 (GH1), P < 0.05 (Fig. 3C); and protein kinase cGMP-dependent 2 (PRKG2), P < 0.01 (Fig. 3D).
Figure 3.
qPCR validation of the RNA sequencing (RNAseq) of upregulated genes from the gastric fundus and corpus. A: acyl-CoA synthetase long-chain family member 5 (ACSL5); B: carbonic anhydrase 12 (CA12); C: growth hormone 1 (GH1); and D: protein kinase cGMP-dependent 2 (PRKG2). Data are presented as means ± SE. n = 24/region. *P < 0.05, **P < 0.01 ***P < 0.001.
Genes of Interest with Overlapping Expression Levels
We also validated expression levels of highly upregulated genes in both the fundus and the corpus of the human stomach. These include chromogranin A (CHGA) (Fig. 4A); gastrokine 1 (GKN1) (Fig. 4B); gastrokine 2 (GKN2) (Fig. 4C); hemoglobin α-globin 1/2 (HBA1/2) (Fig. 4D); joining chain of multimeric IgA and IgM (JCHAIN) (Fig. 4E); lipase F, gastric type (LIPF) (Fig. 4F); latent transforming growth factor β-binding protein 4 (LTBP4) (Fig. 4G); perilipin 1 (PLIN1) (Fig. 4H); transgelin 2 (TAGLN2) (Fig. 4I); trefoil factor 1 (TFF1) (Fig. 4J); transforming growth factor-β 1 (TGFβ1) (Fig. 4K); and vasoactive intestinal peptide (VIP) (Fig. 4L).
Figure 4.
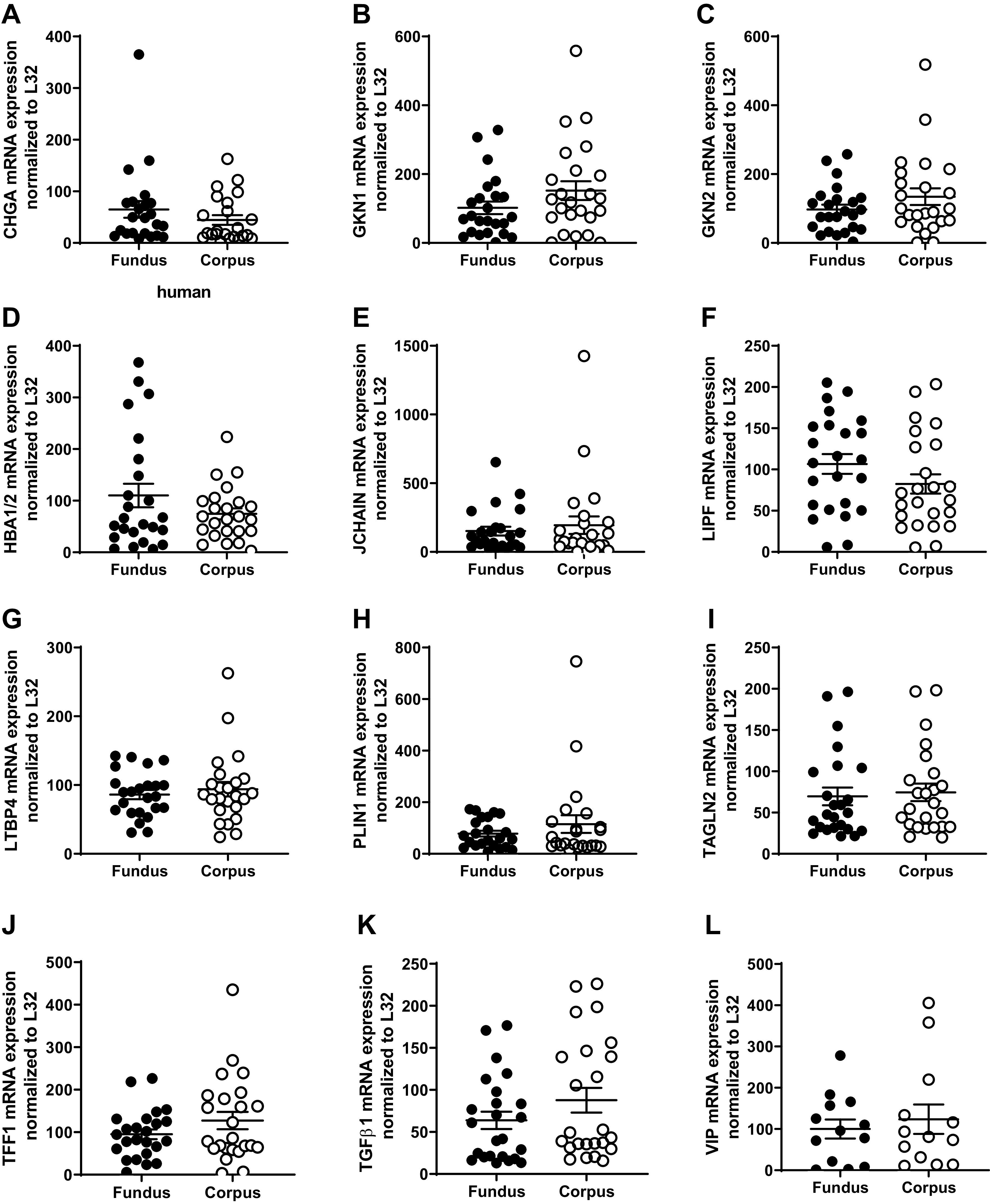
qPCR validation of the RNA sequencing (RNAseq) of upregulated genes using RNA from the gastric fundus and corpus. The following genes were highly expressed in both the fundus and the corpus. A: chromogranin A (CHGA); B: gastrokine 1 (GKN1); C: gastrokine 2 (GKN2); D: hemoglobin α-globin 1/2 (HBA1/2); E: joining chain of multimeric IgA and IgM (JCHAIN); F: lipase F, gastric type (LIPF); G: latent transforming growth factor β-binding protein 4 (LTBP4); H: perilipin 1 (PLIN1); I: transgelin 2 (TAGLN2); J: trefoil factor 1 (TFF1); K: transforming growth factor-β 1 (TGFβ1); and L: vasoactive intestinal peptide (VIP). Data are presented as means ± SE, n = 24 paired samples.
DISCUSSION
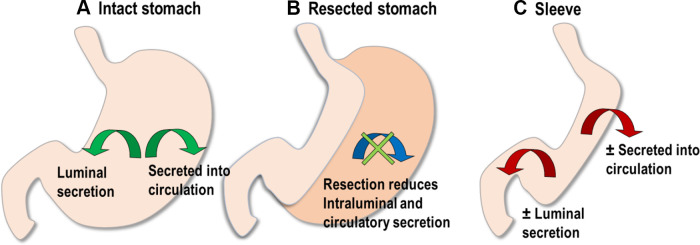
To our knowledge, the current study is the first instance of a complete molecular characterization of the human fundus and corpus collected from the benign obese stomach being reported using a whole transcriptome analysis coupled with validation by real-time PCR. Our hypothesis drives the comparison of fundus and corpus that regional differences exist in the molecular makeup of the human stomach, i.e., it is not homogeneous. Postsurgical outcomes are not produced directly by the loss of excess weight; some are the result of indirect methods. We purport that the differences between various surgical procedures and lack of understanding regarding the regional variations of the proteins of interest in the gastric mucosa may influence the resolution of obesity-related comorbidities. In the intact stomach, the various regions of the stomach produce factors that can influence the local gastric environment or produce effects by luminal secretion or on the whole body through secretion into the circulation (Fig. 5A). In VSG, the resected stomach is no longer present to exert any of its effects (Fig. 5B).
Figure 5.
Rationale for studying the stomach. A: the intact stomach secretes proteins either into circulation or intraluminally. B: vertical sleeve gastrectomy (VSG) results in the removal of the proteins within the greater curvature that may or may not also be expressed in the lesser curvature. C: in the newly fashioned sleeve, a new selection of proteins may have greater (or lesser) influence through luminal or circulatory release.
Given that some variations occur in the amount of fundus or corpus that is resected, these variations could, presumably, produce some variability in metabolic outcomes; there could be a positive benefit due to more (or less) of the stomach producing the particular protein. Alternately, if an actively produced protein in the corpus or the fundus provides a health benefit, resection could be negative, especially to long-term outcomes. This also means that in the remaining stomach, which has been fashioned into the new sleeve, the remaining gastric tissue producing the protein may have a greater (or lesser) influence on physiology depending on the protein’s actions (Fig. 5C). A more precise understanding of these regional differences may allow sparing of stomach areas with higher densities of specific proteins during surgical resection, resulting in retained signaling function, potentially patients’ long-term outcomes. Furthermore, individual variation of the expression of these gastric genes could potentially play an essential role in the individual patient’s postsurgical response in either the acute or the chronic setting. Given that the stomach is often thought to be a sac with homogenous expression, this is not commonly considered.
The rationale for the present work was to identify the molecular constituents of the resected stomach as a foray into identifying candidates that may have a novel influence to produce the resolution of obesity-related comorbidities, such as T2DM, nonalcoholic fatty liver disease (NAFLD), hypertension, and dyslipidemia. These data could also be mined to understand why there could be a lack of improvement in some individuals in the long term. The goal of our discussion is to highlight the role of the genes that we validated. We chose these on the basis of their known roles such as digestion, immunological function, hormone secretion, and cancer diagnosis/prevention, and their potential to impact long-term outcomes.
Digestive Activity and Lipid Processing
Given that digestion is an overwhelmingly important function for the stomach, it is not surprising that many of the critical genes expressed are involved in the mechanical and chemical degradation of nutrient molecules. One of the shortcomings of VSG is the increased incidence of gastroesophageal reflux disease (GERD) and erosive esophagitis (7). This is interesting because carbonic anhydrase (CA), found within the parietal cells of the gastric mucosa, plays an essential role in gastric acid production to acidify the stomach for food digestion (8). The CA12 family member has significantly elevated levels in the corpus compared with the fundus. Resection of CA12 may change the level of expression of the remaining lesser curvature following VSG. On the other hand, acidic chitinase (CHIA), more highly expressed in the fundus, is pivotal for the degradation of chitin found in plant cell walls (9), and CHIA may further provide a digestive defense mechanism against parasitic infections (9). Of all the genes expressed in the stomach, the aspartic protease called progastricsin (PGC) or “pepsinogen C” is the most highly expressed gene in both regions, and even more so in the fundus. PGC is an inactive zymogen produced by the chief cells of the gastric epithelium and is cleaved into active pepsin for the initial breakdown of proteins into polypeptides by the stomach (10).
Perilipin I (PLINI1), also known as lipid droplet-associated protein, is responsible for the formation of a protective coating on the surface of lipid droplets within adipocytes, preserving them against inappropriate lipolysis, until activated by lipolytic signals for appropriate breakdown via hormone-sensitive lipase (11). PLIN1 is primarily expressed in adipose tissue and female breast tissue (8), but in the RNAseq dataset, substantial expression in both the fundus and the corpus was observed. Generally, levels of PLIN1 are reduced in adipocytes of obese individuals compared with those of lean individuals (11). Furthermore, human adipocyte levels of perilipin protein are inversely correlated with lipolytic rate (11), supporting an essential role of PLIN1 in lipolytic regulation in vivo. Although PLIN1 has not been associated with obesity or T2DM in genome-wide association studies, methylation changes in the PLIN1 promotor regions suggest that the regulatory control could be epigenetic (11). The role of PLIN1 in gastric lipolysis has never been addressed in the literature.
Gastric lipase F (LIPF) is secreted by chief cells located in the mucosa of the fundus (12). Gastric lipase is also known as “acid lipase” because it functions at lower pH levels than pancreatic lipases and does not require additional factors such as bile acids or coenzymes for activation (13). In adults, acid lipases are responsible for 30% of lipid hydrolysis (12). Acid lipases like LIPF may provide an alternative means of lipid digestion in times of pancreatic exocrine dysfunction or biliary obstruction. Hence, resection of LIPF in VSG may alter lipid digestion.
Immune System Function
There were a significant number of genes that we probed that could be generally categorized as important to immune system function, namely, GC, transforming growth factor-β 1 (TGFβ1), and JCHAIN. There are varied reports of inflammatory factors that are improved following VSG in circulation. For example, GC is a gene responsible for encoding the transport protein, vitamin D-binding protein (DBP). Although DBP is predominantly localized to the liver and the gall bladder (8), significant expression in the stomach with elevated levels in the gastric fundus was observed. Vitamin D deficiency affects over 1 billion people worldwide (14), and obese individuals are at a greater risk. In a study of obese patients obtaining RYGB, 90% of the patients had vitamin D deficiency, and 100% of the patients were deficient after surgery (15). DBP influences immune function by bolstering the immune response and protects against bacterial invasion (16). Furthermore, it also plays a role in antiproliferative and antitumorigenic activities in the breast, colon, skin, stomach, and prostate (16).
Transforming growth factor-β 1 (TGFβ1) is a versatile cytokine that activates target genes for the regulation of the cell cycle of many cells and promotes chemotaxis of immune cells. Although ubiquitously expressed in the body, the highest level of expression of TGFβ1 is in the bone marrow, spleen, and gastrointestinal tissues (i.e., stomach and small intestine). In our study, transforming growth factor-β 1 (TGFβ1), a versatile cytokine involved in the regulation of the cell cycle and immune cell chemotaxis, was expressed highly in the fundus and the corpus. TGFβ levels rise with increasing adiposity, and following surgical weight loss, TGFβ levels decline (17–19), but this may be partially through resection of the TGFβ-producing gastric cells. On the other hand, latent TGFβ-binding protein 4 (LTBP4) is highly expressed in the greater curvature and regulates TGFβ binding to suppress its activity.
Joining chain of multimeric IgA and IgM (JCHAIN) codes for an important linking protein between immunoglobulin isotypes IgA and IgM, and it is highly expressed in the GI tract and immune system (8) in both the fundus and corpus regions. JCHAIN functions as a link to form IgA dimers secreted into circulation and taken up by endothelial cells, such as those lining the gut, where it gains a protective secretory component and is released into the intestinal lumen to fight enteric infections (20). Significant resection of the JCHAIN could presumably reduce the effectiveness of its protection of the GI tract.
Hormonal Secretion
The stomach is replete with a vast number of gastric hormones that are highly expressed in the greater curvature. Among the ones we validated are ghrelin (GHRL), growth hormone 1 (GH1), somatostatin (SST), and vasoactive intestinal peptide (VIP).
GHRL is a 28-amino-acid peptide primarily secreted from the gastric fundus and by proximal small intestinal mucosa (21). GHRL is known as the “hunger hormone” because of its precipitous rise before a meal and reduction directly following food intake (21). Serum ghrelin levels in obese individuals are reduced compared with in lean individuals (22). Much debate has occurred over the role of ghrelin in driving the improvements to both body weight regulation and insulin sensitivity following surgical weight loss (6, 23). Resection of GHRL in VSG results in a significant reduction in circulating ghrelin (6, 24, 25). Differences in long-term outcomes in rodents and humans obtaining bariatric surgery have resulted in a controversy surrounding the interplay of ghrelin resection and surgical weight reduction.
GH is most highly expressed in the pituitary gland’s anterior lobe and is vital for appropriate development of the body (26); GH secretion is also stimulated by ghrelin, which is produced mainly in the stomach, linking GH with GI function (26), but its role and presence in the stomach is not understood. GH1 mediates most of its anabolic effects indirectly by stimulating the hepatic production and release of insulin-like growth factor 1 (26). GH function is diabetogenic, as it opposes the effects of insulin, increases serum glucose, and is elevated in patients with T2DM and proliferative diabetic retinopathy and diabetic nephropathy (27). GH and IGF-1 levels are dysregulated in obesity, and obesity-induced dysregulation of GH is reversed with weight loss, emphasizing the potential therapeutic role of bariatric surgery in treating obesity comorbidities (28). It is unknown how the resection of gastric GH contributes to the improvement of obesity comorbidities.
SST is found throughout the body but most notably in the GI tract, brain, pancreas, and adrenal glands (8). Similar to GHRL, its expression is significantly higher in the fundus compared with the corpus. In particular, D cells located in both the gastric mucosa and pancreatic islets secrete somatostatin, which acts to inhibit several secretory proteins necessary for appropriate gastrointestinal functioning and nutrient absorption, such as insulin and glucagon from the pancreas, gastrin, secretin, CCK, VIP, GLP-1, gastric inhibitory peptide, ghrelin, pepsin, and gastric acid from the digestive tract, as well as several hormones secreted from the brain, thyroid, and adrenals (29). The majority of gastrointestinal D cells are found scattered throughout the stomach, contributing to 65% of total body somatostatin (30). Although our knowledge about the exact signals that influence gastric D cell somatostatin release is currently limited, studies have shown that nutrient sensors are present on these cells, indicating the direct influence of diet on somatostatin regulation (29). In addition, receptors for neuropeptides and neuroendocrine hormones are also present on D cells, emphasizing the complex interplay of physiological mediators in gastrointestinal somatostatin secretion (29). Therefore, resection of the greater curvature would reduce SST contribution from the stomach and decrease the whole body pool of somatostatin in VSG. No studies on the contribution of somatostatin after VSG have been performed.
VIP encodes an important gastrointestinal neuropeptide hormone secreted by neurons of the central, peripheral, and enteric nervous system with both local intestinal and systemic activity. VIP affects a wide variety of gastrointestinal functions, such as regulation of gastric acid secretion, intestinal secretion of fluid and bicarbonate, pancreatic enzyme release, cellular motility and proliferation, vascular vasodilation, and relaxation of intestinal smooth muscle and sphincters (31). Elevated abnormal VIP release, along with an increase in mast cell function, has also been implicated in irritable bowel syndrome with diarrhea (31). VIP is also thought to have antibacterial properties and prevent intestinal barrier disruption (32). Given the immense role of VIP GI signaling, immunomodulation, and barrier function, it is unknown whether resection of the stomach VIP influences VIP expression in other areas of the GI tract to compensate for its diminished presence.
Cancer Detection, Progression, and Suppression
A significant number of gastric genes that we investigated are associated with tumor growth and cancer progression. The risk of developing gastrointestinal cancers is markedly higher in obese patients compared with normal-weight individuals (33). Furthermore, obtaining bariatric surgery reduces overall cancer risk compared with controls who do not receive surgery (34). Improvements to excess body weight and obesity-related comorbidities may directly reduce the incidence of cancer in this population. However, it is also possible that the resection of parts of the stomach that produce highly carcinogenic proteins may be responsible for the reduced cases of malignancy in obese individuals seeking bariatric treatments.
For example, CA12, significantly elevated in the corpus, is a tumor marker for renal clear cell carcinoma, breast tumors, and astrocytic gliomas in the brain (35). The CA12-5 variant has specifically emerged for its role as a potential biomarker for gastric cancers (36). PGC has been identified as a biomarker for non-gastrointestinal cancers since its ectopic secretion is elevated in sex-hormone producing tumors. Removal of PGC-containing stomach as a result of VSG may reduce the use of this enzyme as a negative marker for cancer progression.
The REG1A gene is also known as lithostathine-1α, islet cells regeneration factor (ICRF), or islet of Langerhans regenerating protein (37, 38); it is highly expressed in the pancreas and less so in the gastrointestinal tract (8). Here, we found significantly greater levels of REG1A in the fundus compared with the corpus. REG1A and its associated family members were also identified to cause regeneration of pancreatic β cells when upregulated locally (39). The REG1A gene has also been found to be upregulated in both inflammatory bowel disease and colorectal cancer (40) and plays an important role in other cancers (41).
GKN1/2 are gastric mucosal secretory proteins almost exclusively produced in the stomach (8). Gastrokines are believed to promote intestinal and colonic health and produce anti-inflammatory effects in the GI tract. GKN1/2 interact with another tumor suppressor, TFF1, one of the most highly expressed genes in the stomach, to maintain gut homeostasis and growth of the gastric mucosa (42). GKN1 appears to inhibit gastrin-induced epithelial proliferation in both healthy and cancerous gastric tissues, further supporting its role in gastric epithelial homeostasis, tissue repair, and tumor suppression (43). Resection of these gastrokines may potentially place bariatric surgery patients at a long-term disadvantage due to the purported protective influence of gastrokines in the stomach. Currently, gastric cancer occurrences within 10 years post-VSG have been reported minimally through case reports only (44).
PRKG2, an intracellular cGMP-dependent protein kinase highly expressed in the human GI tract and in our sample set, was upregulated in the corpus. Studies in mice found that a deficiency of PRKG2 predisposed the small intestinal crypts of Lieberkühn to tumorigenesis (45), and a separate study showed ectopic PRKG2 inhibited proliferation of mouse colonic cancer cells (46), providing further evidence for the antiproliferative effects of PRKG2.
LTBP4, which regulates TGFβ binding to suppress its activity, is downregulated in adenocarcinomas and squamous cell carcinomas of the esophagus both in vitro and in vivo (47). Furthermore, adenocarcinomas of the stomach, pancreas, and small intestine all have significantly repressed levels of LTPB4 compared with healthy tissue controls, suggesting that the loss of LTPB4 function may permit cancer progression (48). In the case of LTPB4, which appears to actively protect against cancer, resection of its presence in the stomach may have a negative effect on cancer susceptibility.
Other Genes of Unique Function
There were several genes of varying functions that we validated that did not fit into our overall categorization schema but are worth noting, in particular IRX5.
IRX5 is a transcription factor expressed in a variety of tissue types and, in particular, elevated in our dataset in the fundus. Interestingly, the IRX gene cluster has been localized just downstream to the FTO (fat mass and obesity-associated protein) gene locus on chromosome 16, which is known to have a strong genetic association with BMI in humans (49) and independent associations with T2DM (50). Obese bariatric patients were evaluated to determine the role of FTO variants [either no high-risk (TT) allele, one high-risk (TA), or two high-risk (AA) alleles] on surgical outcomes (51). Interestingly, 71.2% of the bariatric surgery candidates carried at least one high-risk allele, and beginning 36 months after the surgery, weight and BMI increases were evident in the allele carriers compared with the noncarriers, indicating a potential role of FTO genetic variants effect on bariatric surgery outcomes (51). Given the proximity of the IRX gene locus and FTO, it would be of great interest to compare gastric IRX gene’s expression in obese bariatric candidates versus healthy lean patients in future studies.
Caveats, Strengths, Considerations, and Significance
There are several considerations specific to the current study that must be accounted for in light of our overall findings. This study utilized the resected stomach samples of patients undergoing elective VSG for obesity and its associated comorbidities. A layer of important information is missing because we were not able to obtain samples from similar regions in healthy nonobese individuals to assess for variation in expression of the target genes based on BMI and level of adiposity. Individual variations existed in the sizes of the participant’s stomach; thus, the exact location of the fundus and corpus biopsy could not be controlled for. In the current study, RNA was isolated from all layers of the stomach (mucosal, submucosa, muscularis, and serosa). It is possible that individual variation in the thickness of the tissue layers may have altered gene expression patterns. In the future, endoscopic biopsies would limit the sampling to the mucosal layer, and thus, more controlled sampling could be obtained. Alternately, single-cell RNA sequencing could be performed on whole tissue disassociation.
A single surgeon performed each bariatric procedure with an identical technique in our study, specifically by laparoscopic VSG, thus reducing technical variability. Hence, all of the patients received similar perioperative and postoperative regimens. Each participant was fasted and prepped for surgery before obtaining samples; therefore, a transient change in expression from the baseline may have occurred. We view controlled presurgical fasting as a strength in our study because all samples were normalized by the timing of the last meal.
The majority of the patient population in our study were White and female, with the only minority sample from African American ancestry. Future studies are warranted to investigate further the potential contribution of race and gender in gastric gene expression. Regarding gender differences, the number of females obtaining bariatric surgery in the United States is far greater than males. Current studies have shown that bariatric surgery participants consist of 80% females and 20% males (52). Since our study participants were predominantly female and given this unbalanced ratio of participants nationally, we cannot report as many males, which is an essential parameter to follow-up in future studies regarding gender differences.
Concluding Statement
A myriad of genes expressed in the region of the resected stomach in VSG display a multiplicity of functions yet to be described. Unearthing these genes and their physiological roles is crucial for understanding how limiting their expression post-VSG leads to early improvement or resolution of obesity-related comorbidities. Although the impact of VSG on long-term health outcomes and remission of chronic disease is not entirely known, therapies aiming to inhibit these genetic targets may provide noninvasive and reversible alternatives to treat obesity effectively.
GRANTS
B.E.G. is supported by awards from the Office of the Assistant Secretary of Defense for Health Affairs supported by Award No. W81XWH-16-1-0349 and W81XWH-16-1-0387. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. The work performed through the UMMC Molecular and Genomics Facility is supported, in part, by funds from the NIGMS, including Mississippi INBRE (P20GM103476), Obesity, Cardiorenal and Metabolic Diseases-COBRE (P20GM104357), and Mississippi Center of Excellence in Perinatal Research (MS-CEPR)-COBRE (P20GM121334).
DISCLAIMERS
The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.C.D., M.R.G., B.A.W., K.D.V., and B.E.G. conceived and designed research; W.C.D., M.R.G., B.A.W., W.J.L., A.R.H., A.D., K.D.V., and B.E.G. performed experiments; W.C.D., M.R.G., B.A.W., W.J.L., and B.E.G. analyzed data; W.C.D., M.R.G., and B.E.G. interpreted results of experiments; W.C.D., M.R.G., and B.E.G. prepared figures; W.C.D., M.R.G., and B.E.G. drafted manuscript; W.C.D., M.R.G., B.A.W., W.J.L., and B.E.G. edited and revised manuscript; W.C.D., M.R.G., B.A.W., W.J.L., A.R.H., A.D., K.D.V., and B.E.G. approved final version of manuscript.
REFERENCES
- 1.Courcoulas AP, Gallagher JW, Neiberg RH, Eagleton EB, DeLany JP, Lang W, Punchai S, Gourash W, Jakicic JM. Bariatric surgery vs. lifestyle intervention for diabetes treatment: five year outcomes from a randomized trial. J Clin Endocrinol Metab 105: 866–876, 2020. doi: 10.1210/clinem/dgaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjostrom L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, Ahlin S, Anveden Å, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lönroth H, Narbro K, Näslund I, Olbers T, Svensson P-A, Carlsson LMS. Bariatric surgery and long-term cardiovascular events. JAMA 307: 56–65, 2012. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 3.ASMBS. Estimate of Bariatric Surgery Numbers, 2011–2018. American Society for Metabolic Bariatric Surgery, 2018. [Google Scholar]
- 4.Baumann T, Kuesters S, Grueneberger J, Marjanovic G, Zimmermann L, Schaefer A-O, Hopt UT, Langer M, Karcz WK. Time-resolved MRI after ingestion of liquids reveals motility changes after laparoscopic sleeve gastrectomy—preliminary results. Obes Surg 21: 95–101, 2011. doi: 10.1007/s11695-010-0317-6. [DOI] [PubMed] [Google Scholar]
- 5.Roslin MS, Dudiy Y, Weiskopf J, Damani T, Shah P. Comparison between RYGB, DS, and VSG effect on glucose homeostasis. Obes Surg 22: 1281–1286, 2012. doi: 10.1007/s11695-012-0686-0. [DOI] [PubMed] [Google Scholar]
- 6.Alamuddin N, Vetter ML, Ahima RS, Hesson L, Ritter S, Minnick A, Faulconbridge LF, Allison KC, Sarwer DB, Chittams J, Williams NN, Hayes MR, Loughead JW, Gur R, Wadden TA. Changes in fasting and prandial gut and adiposity hormones following vertical sleeve gastrectomy or Roux-en-Y-gastric bypass: an 18-month prospective study. Obes Surg 27: 1563–1572, 2017. doi: 10.1007/s11695-016-2505-5. [DOI] [PubMed] [Google Scholar]
- 7.Lim CH, Lee PC, Lim E, Tan J, Chan WH, Tan HC, Ganguly S, Tham KW, Eng A. Correlation between symptomatic gastro-esophageal reflux disease (GERD) and erosive esophagitis (EE) post-vertical sleeve gastrectomy (VSG). Obes Surg 29: 207–214, 2019. doi: 10.1007/s11695-018-3509-0. [DOI] [PubMed] [Google Scholar]
- 8.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, MardinogluA, et al. Proteomics. Tissue-based map of the human proteome. Science 347: 1260419, 2015. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 9.Vega K, Kalkum M. Chitin, chitinase responses, and invasive fungal infections. Int J Microbiol 2012: 920459, 2012. doi: 10.1155/2012/920459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen S, Jiang J, Yuan Y. Pepsinogen C expression, regulation and its relationship with cancer. Cancer Cell Int 17: 57, 2017. doi: 10.1186/s12935-017-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bialesova L, Kulyté A, Petrus P, Sinha I, Laurencikiene J, Zhao C, Wright KD, Arner P, Dahlman I. Epigenetic regulation of PLIN 1 in obese women and its relation to lipolysis. Sci Rep 7: 10152, 2017. doi: 10.1038/s41598-017-09232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong Y, Zheng Y, Jia Y, Li P, Wang Y. Decreased LIPF expression is correlated with DGKA and predicts poor outcome of gastric cancer. Oncol Rep 36: 1852–1860, 2016. doi: 10.3892/or.2016.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomasik P, Wędrychowicz A, Rogatko I, Zając A, Fyderek K, Sztefko K. Gastric lipase secretion in children with gastritis. Nutrients 5: 2924–2932, 2013. doi: 10.3390/nu5082924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahota O. Understanding vitamin D deficiency. Age Ageing 43: 589–591, 2014. doi: 10.1093/ageing/afu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson LA, Cheskin LJ, Furtado M, Papas K, Schweitzer MA, Magnuson TH, Steele KE. Malnutrition in bariatric surgery candidates: multiple micronutrient deficiencies prior to surgery. Obes Surg 26: 833–838, 2016. doi: 10.1007/s11695-015-1844-y. [DOI] [PubMed] [Google Scholar]
- 16.Sirajudeen S, Shah I, Menhali AA. A narrative role of vitamin D and its receptor: with current evidence on the gastric tissues. Int J Mol Sci 20: 3832, 2019. doi: 10.3390/ijms20153832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fain JN, Tichansky DS, Madan AK. Transforming growth factor β1 release by human adipose tissue is enhanced in obesity. Metabolism 54: 1546–1551, 2005. doi: 10.1016/j.metabol.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Cottam DR, Mattar SG, Barinas-Mitchell E, Eid G, Kuller L, Kelley DE, Schauer PR. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg 14: 589–600, 2004. doi: 10.1381/096089204323093345. [DOI] [PubMed] [Google Scholar]
- 19.Porreca E, Di Febbo C, Vitacolonna E, Baccante G, Castelnuovo A, Angelini A, Febo F, Di Nisio M, Cuccurullo F. Transforming growth factor-β1 levels in hypertensive patients: association with body mass index and leptin. Am J Hypertens 15: 759–765, 2002. doi: 10.1016/S0895-7061(02)02978-3. [DOI] [PubMed] [Google Scholar]
- 20.Xiong E, Li Y, Min Q, Cui C, Liu J, Hong R, Lai N, Wang Y, Sun J, Matsumoto R, Takahashi D, Hase K, Shinkura R, Tsubata T, Wang J-Y. MZB1 promotes the secretion of J-chain-containing dimeric IgA and is critical for the suppression of gut inflammation. Proc Natl Acad Sci USA 116: 13480–13489, 2019. doi: 10.1073/pnas.1904204116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, ArgenteJ, et al. Ghrelin. Mol Metab 4: 437–460, 2015. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S-I, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 87: 240–244, 2002. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- 23.Nosso G, Griffo E, Cotugno M, Saldalamacchia G, Lupoli R, Pacini G, Riccardi G, Angrisani L, Capaldo B. Comparative effects of Roux-en-Y gastric bypass and sleeve gastrectomy on glucose homeostasis and incretin hormones in obese type 2 diabetic patients: a one-year prospective study. Horm Metab Res 48: 312–317, 2016. doi: 10.1055/s-0041-111505. [DOI] [PubMed] [Google Scholar]
- 24.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab 89: 2608–2615, 2004. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 25.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623–1630, 2002. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 26.Soendergaard C, Young JA, Kopchick JJ. Growth hormone resistance—special focus on inflammatory bowel disease. Int J Mol Sci 18: 1019, 2017. doi: 10.3390/ijms18051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu M, Flanagan JU, Langley RJ, Hay MP, Perry JK. Targeting growth hormone function: strategies and therapeutic applications. Signal Transduct Target Ther 4: 3, 2019. doi: 10.1038/s41392-019-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornejo-Pareja I, Clemente-Postigo M, Tinahones FJ. Metabolic and endocrine consequences of bariatric surgery. Front Endocrinol (Lausanne) 10: 626, 2019. doi: 10.3389/fendo.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mani BK, Zigman JM. A strong stomach for somatostatin. Endocrinology 156: 3876, 2015. doi: 10.1210/en.2015-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rorsman P, Huising MO. The somatostatin-secreting pancreatic δ-cell in health and disease. Nat Rev Endocrinol 14: 404–414, 2018. doi: 10.1038/s41574-018-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwasaki M, Akiba Y, Kaunitz JD. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: focus on the gastrointestinal system. F1000Res 8: F1000 Faculty Rev-1629, 2019. doi: 10.12688/f1000research.18039.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conlin VS, Wu X, Nguyen C, Dai C, Vallance BA, Buchan AMJ, Boyer L, Jacobson K. Vasoactive intestinal peptide ameliorates intestinal barrier disruption associated with Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol 297: G735–G750, 2009. doi: 10.1152/ajpgi.90551.2008. [DOI] [PubMed] [Google Scholar]
- 33.Karczewski J, Begier-Krasińska B, Staszewski R, Popławska E, Gulczynska-Elhadi K, Dobrowolska A. Obesity and the risk of gastrointestinal cancers. Dig Dis Sci 64: 2740–2749, 2019. doi: 10.1007/s10620-019-05603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghavendra RS, Kini D. Benign, premalignant, and malignant lesions encountered in bariatric surgery. JSLS 16: 360–372, 2012. doi: 10.4293/108680812X13462882736457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waheed A, Sly WS. Carbonic anhydrase XII functions in health and disease. Gene 623: 33–40, 2017. doi: 10.1016/j.gene.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen C, Chen Q, Zhao Q, Liu M, Guo J. Value of combined detection of serum CEA, CA72-4, CA19-9, CA15-3 and CA12-5 in the diagnosis of gastric cancer. Ann Clin Lab Sci 47: 260–263, 2017. [PubMed] [Google Scholar]
- 37.Okamoto H. The Reg gene family and Reg proteins: with special attention to the regeneration of pancreatic β-cells. J Hepatobiliary Pancreat Surg 6: 254–262, 1999. doi: 10.1007/s005340050115. [DOI] [PubMed] [Google Scholar]
- 38.Akiyama T, Takasawa S, Nata K, Kobayashi S, Abe M, Shervani NJ, Ikeda T, Nakagawa K, Unno M, Matsuno S, Okamoto H. Activation of Reg gene, a gene for insulin-producing beta-cell regeneration: poly(ADP-ribose) polymerase binds Reg promoter and regulates the transcription by autopoly(ADP-ribosyl)ation. Proc Natl Acad Sci USA 98: 48–53, 2001. doi: 10.1073/pnas.98.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe T, Yonemura Y, Yonekura H, Suzuki Y, Miyashita H, Sugiyama K, Moriizumi S, Unno M, Tanaka O, Kondo H. Pancreatic beta-cell replication and amelioration of surgical diabetes by Reg protein. Proc Natl Acad Sci USA 91: 3589–3592, 1994. doi: 10.1073/pnas.91.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sekikawa A, Fukui H, Fujii S, Nanakin A, Kanda N, Uenoyama Y, Sawabu T, Hisatsune H, Kusaka T, Ueno S, Nakase H, Seno H, Fujimori T, Chiba T. Possible role of REG Iα protein in ulcerative colitis and colitic cancer. Gut 54: 1437–1444, 2005. doi: 10.1136/gut.2004.053587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu Y-S, Liao G-J, Jiang N-N. DNA methylation-mediated silencing of regenerating protein 1 alpha (REG1A) affects gastric cancer prognosis. Med Sci Monit 23: 5834–5843, 2017. doi: 10.12659/MSM.904706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menheniott TR, Kurklu B, Giraud AS. Gastrokines: stomach-specific proteins with putative homeostatic and tumor suppressor roles. Am J Physiol Gastrointest Liver Physiol 304: G109–G121, 2013. doi: 10.1152/ajpgi.00374.2012. [DOI] [PubMed] [Google Scholar]
- 43.Kim O, Yoon JH, Choi WS, Ashktorab H, Smoot DT, Nam SW, Lee JY, Park WS. Gastrokine 1 inhibits gastrin-induced cell proliferation. Gastric Cancer 19: 381–391, 2016. doi: 10.1007/s10120-015-0483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho SM, Lee S, Yang S-H, Kim HY, Lee MJ, Kim HV, Kim J, Baek S, Yun J, Kim D, Kim YK, Cho Y, Woo J, Kim TS, Kim YS. Age-dependent inverse correlations in CSF and plasma amyloid-β(1-42) concentrations prior to amyloid plaque deposition in the brain of 3xTg-AD mice. Sci Rep 6: 20185, 2016. doi: 10.1038/srep20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li P, Schulz S, Bombonati A, Palazzo JP, Hyslop TM, Xu Y, Baran AA, Siracusa LD, Pitari GM, Waldman SA. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology 133: 599–607, 2007. doi: 10.1053/j.gastro.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 46.Wang R, Kwon I-K, Thangaraju M, Singh N, Liu K, Jay P, Hofmann F, Ganapathy V, Browning DD. Type 2 cGMP-dependent protein kinase regulates proliferation and differentiation in the colonic mucosa. Am J Physiol Gastrointest Liver Physiol 303: G209–G219, 2012. doi: 10.1152/ajpgi.00500.2011. [DOI] [PubMed] [Google Scholar]
- 47.Bultmann I, Conradi A, Kretschmer C, Sterner-Kock A. Latent transforming growth factor β-binding protein 4 is downregulated in esophageal cancer via promoter methylation. PLoS One 8: e65614, 2013. doi: 10.1371/journal.pone.0065614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mauri G, Sartore-Bianchi A, Russo A-G, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol 13: 109–131, 2019. doi: 10.1002/1878-0261.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rask-Andersen M, Almen MS, Schioth HB. Scrutinizing the FTO locus: compelling evidence for a complex, long-range regulatory context. Hum Genet 134: 1183–1193, 2015. doi: 10.1007/s00439-015-1599-5. [DOI] [PubMed] [Google Scholar]
- 50.Bjune J-I, Haugen C, Gudbrandsen O, Nordbø OP, Nielsen HJ, Våge V, Njølstad PR, Sagen JV, Dankel SN, Mellgren G. IRX5 regulates adipocyte amyloid precursor protein and mitochondrial respiration in obesity. Int J Obes (Lond) 43: 2151–2162, 2019. doi: 10.1038/s41366-018-0275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodrigues GK, Resende CMM, Durso DF, Rodrigues LAA, Silva JLP, Reis RC, Pereira SS, Ferreira DC, Franco GR, Alvarez-Leite J. A single FTO gene variant rs9939609 is associated with body weight evolution in a multiethnic extremely obese population that underwent bariatric surgery. Nutrition 31: 1344–1350, 2015. doi: 10.1016/j.nut.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 52.Kochkodan J, Telem DA, Ghaferi AA. Physiologic and psychological gender differences in bariatric surgery. Surg Endosc 32: 1382–1388, 2018. doi: 10.1007/s00464-017-5819-z. [DOI] [PubMed] [Google Scholar]