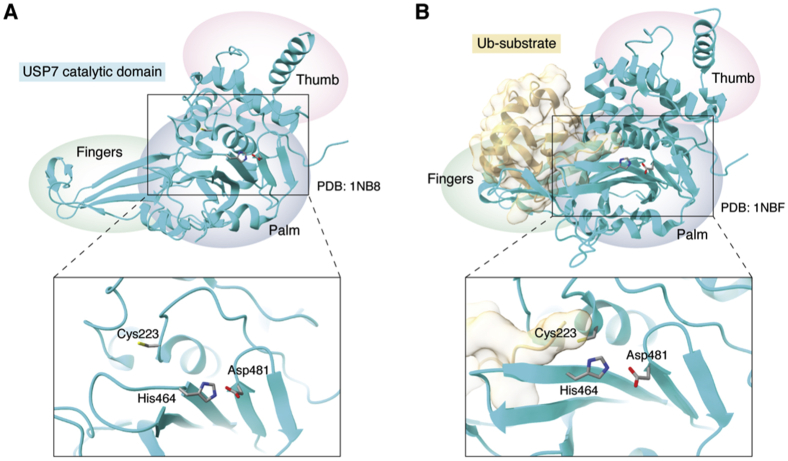
Figure 3.
Conformations of cysteine proteases. Inactive (A, PDB: 1NB8) and active (B, PDB: 1NBF) conformations of the USP family cysteine protease USP7 are shown in cyan. Below are enlarged views of the catalytic triad positions. In the inactive state (A), the catalytic cysteine (Cys223) is positioned far from the other members of the catalytic triad (His464 and Asp481). This prevents the histidine from lowering the pKa to deprotonate the thiol of the cysteine and promote the active state. In the active state (B), USP7 is bound to a ubiquitin substrate, which induces a conformational shift that brings the catalytic cysteine closer to the other members of the catalytic triad, enabling its deprotonation into a reactive thiolate. In either case, the fingers domain (containing the ubiquitin interaction motif), as well as the palm and thumb domains (containing the catalytic center) are indicated.