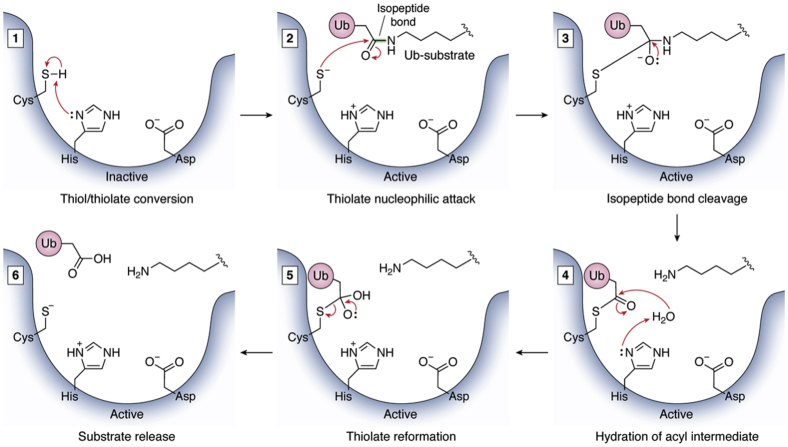
Figure 4.
DUB cysteine protease catalytic mechanism. A generalized mechanism of cysteine protease DUBs is shown, including the three most common members of the catalytic triad (Cys, His, and Asp) and the isopeptide bond of the ubiquitinated substrate. The general steps of the mechanism are as follows: 1, the histidine, depolarized by the aspartate, deprotonates the cysteine, converting its side chain from an inactive thiol to a reactive thiolate. 2, the thiolate of cysteine undergoes a nucleophilic attack on the acyl group of the ubiquitin isopeptide bond, forming a tetrameric intermediate. 3, the isopeptide bond is cleaved as the amide group of the isopeptide bond deprotonates the DUB histidine, freeing the substrate from ubiquitin, which is still bound as an intermediate with the DUB cysteine. 4, hydration of the DUB cysteine acyl intermediate utilizing a water molecule to convert the acyl intermediate to a carboxyl intermediate. 5, the intermediate bond between the ubiquitin and DUB cysteine is broken, reforming the ubiquitin monomer and thiolate. 6, the ubiquitin monomer and substrate are released from the DUB.