Figure 4.
Chronic optogenetic induction of LTD drives pathological hyperphosphorylation of tau
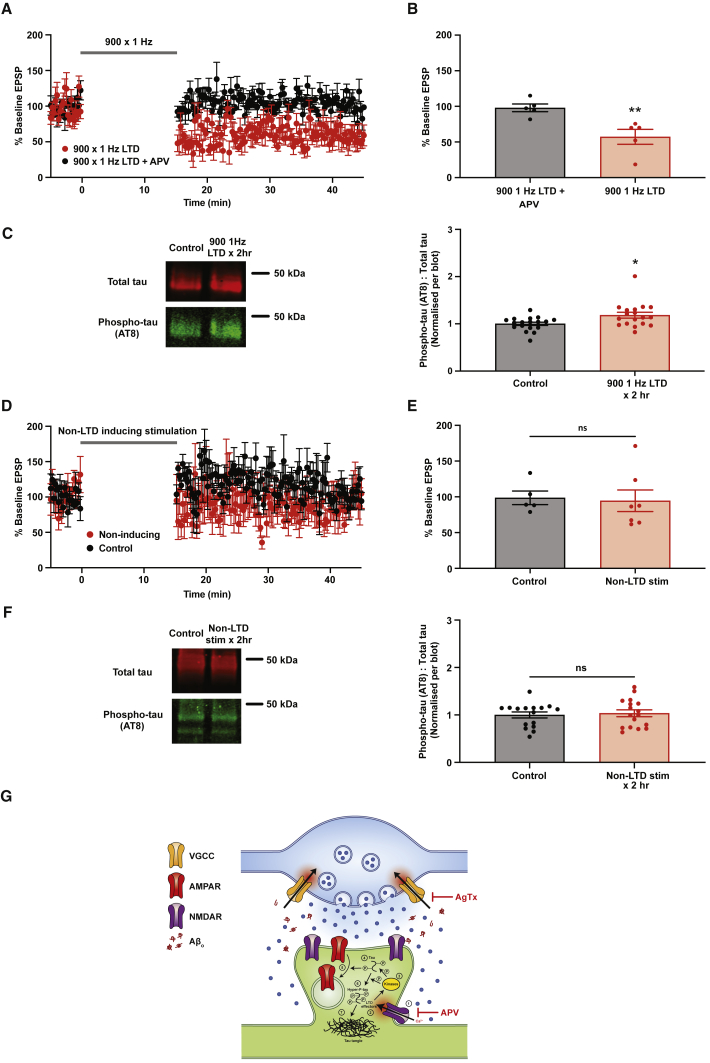
(A) Summary traces of patch-clamp recordings showing slope of EPSP at CA3-CA1 synapses in ChR2-expressing slices following 900 × 1 Hz optical stimulation, with or without the NMDAR antagonist APV as indicated. Traces normalized to pre-stimulation baseline.
(B) Mean average EPSP slopes calculated within a 25- to 30-min time window after LTD induction (900 × 1 Hz: n = 5, 57.24% ± 10.46%; 900 × 1 Hz + APV: n = 5, 97.91% ± 5.36%). Mann-Whitney test.
(C) Western blot analysis of hippocampal slices treated for 7 days as indicated. Left panels show representative bands. The ratio of pathologically phosphorylated tau (AT8 antibody) to total tau was quantified and normalized to control within each blot (control: n = 18, 1.00 ± 0.03; 900 × 1 Hz LTD every 2 h: n = 18, 1.18 ± 0.06).
(D) Summary traces showing slope of EPSP at CA3-CA1 synapses in ChR2-expressing slices following optical stimulation with a non-LTD-inducing stimulus (three stimuli at 3 Hz, repeated every 3 s for 15 min). Traces normalized to pre-stimulation baseline.
(E) Mean average EPSP slopes calculated within a 25- to 30-min time window after delivery of non-inducing stimulus (control: n = 5, 98.61% ± 9.55%; non-LTD-inducing stimulus: n = 7, 94.52% ± 15.10%). Mann-Whitney test.
(F) Western blot analysis of hippocampal slices treated for 7 days as indicated. Non-LTD-inducing stimulation is three stimuli at 3 Hz, repeated every 3 s for 15 min, repeated every 2 h. Left panels show representative bands. The ratio of pathologically phosphorylated tau (AT8 antibody) to total tau was quantified and normalized to control within each blot (control: n = 16, 1.00 ± 0.06; non-LTD-inducing stimulus every 2 h: n = 17, 1.04 ± 0.07).
(G) Schematic diagram showing proposed mechanism of Aβo-induced tau hyperphosphorylation. Aβo enhance the probability of neurotransmitter release from the presynaptic terminal, resulting in increased low-frequency synaptic activity and/or activation of extrasynaptic NMDAR (1), thus promoting the induction of NMDAR-dependent LTD. LTD is initiated by an NMDAR-dependent Ca2+ influx that activates a variety of LTD effector proteins (2) that in turn activate LTD-associated kinases (3). During the physiological induction of LTD, these kinases phosphorylate tau (4), which alters its affinity for microtubules and helps to promote endocytosis and internalization of synaptic AMPAR (5). If the LTD induction stimulus is excessive or unusually prolonged, as a result of pathologically increased synaptic activity, kinase activation may be inappropriately sustained, potentially leading to hyperphosphorylation of tau at non-physiological residues (6). Hyperphosphorylated tau is in itself toxic and will eventually form stable aggregates that give rise to the histopathological inclusions (dystrophic neurites and neurofibrillary tangles) that are diagnostic of AD (7). Note that normalization of neurotransmitter release properties with a low dose of AgTx prevents the excessive induction of LTD and hyperphosphorylation of tau. Error bars represent ± SEM. ∗p < 0.05, ∗∗p < 0.01.