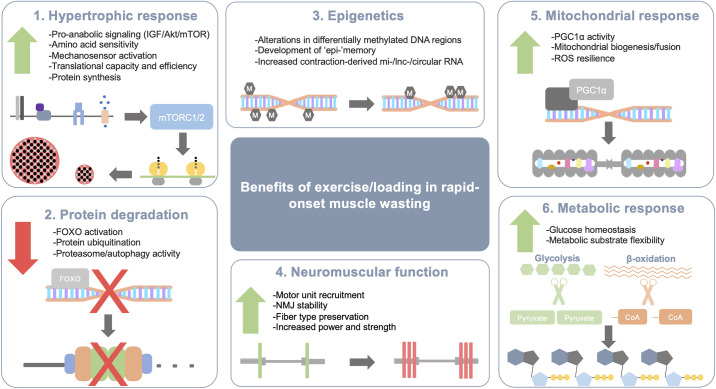
Figure 2.
Range of benefits of exercise for pathophysiology during rapid onset muscle wasting. Acute and chronic exercise training involves the interaction of multiple molecular systems that act together to improve contractile and metabolic function. 1) Activation of pro-anabolic signaling pathways as a result of exercise converges upon mTORC1/2 to increase ribosomal initiation and efficiency to increase protein synthesis. 2) The activation of mTORC1/2 and related anabolic pathways inhibit activation of transcription factors that begin the muscle atrophy program (e.g., FOXO family) to prevent excess protein degradation. When protein synthesis exceeds protein degradation over time, appreciable muscle mass can be gained. 3) Exercise can create long-standing changes in the epigenetic profile of muscle by hypermethylating DNA in promoter regions of deleterious genes to limit their expression while also demethylating DNA regions to increase transcription of beneficial genes. Contraction-mediated increases in noncoding RNA species may regulate gene expression to limit factors that induce muscle wasting. 4) Improved neuromuscular function can result in protected or increased muscle strength and power. Strength and power are two critical clinical outcomes that may be a result of exercise training even if there is no gain in muscle mass. 5) A primary result of exercise is improved mitochondrial density and function. This is mostly mediated by PGC1α nuclear translocation and transcription of key proteins needed for oxidative phosphorylation and mitochondrial fusion. 6) Muscle contractions and sustained exercise require ample amounts of ATP. Events that result in muscle wasting can create metabolic hurdles to proper energy regulation such as insulin resistance. Exercise can improve glucose uptake regardless of insulin sensitivity to help control systemic glucose homeostasis while also using additional fuels such as fatty acids to generate substrate for oxidative phosphorylation and ATP production. lnc, long noncoding; miRNA, microRNA; mTORC1/2, mechanistic target of rapamycin complex 1/2; NMJ, neuromuscular junction; PGC1α, PPARG coactivator 1 alpha; ROS, reactive oxygen species.