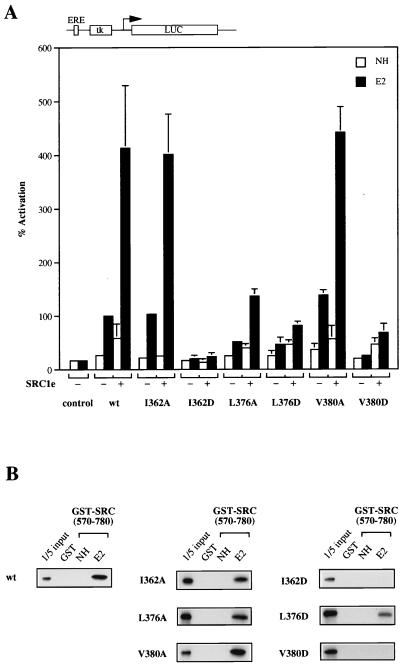
FIG. 2.
Surface hydrophobic residues in helices 3 and 5 of mERα are involved in coactivator binding. (A) COS-1 cells were transiently transfected with expression vector for wild-type (wt) or mutant receptors and pERE-tk-GL3 reporter in the absence (−) or presence (+) of 100 ng of full-length SRC1e. A cytomegalovirus promoter-driven pJ7-lacZ plasmid was cotransfected as the internal control. After transfection, cells were treated with ethanol vehicle alone (NH) or 17β-estradiol (E2) at 10−8 M for 24 h. Subsequently, cells were assayed for luciferase (LUC) and β-galactosidase activity. Normalized values are expressed as percentage of activity compared with that of wild-type mERα alone in the presence of β-estradiol (100%). The results shown represent the average of at least two independent experiments assayed in duplicate + standard errors. ERE, estrogen response element. (B) Binding activity of wild-type or mutant receptors to SRC1 in GST pull-down assay. In vitro-translated, [35S]methionine-labelled receptors were incubated with GST-SRC1 (aa 570 to 780) coupled to Sepharose beads in either the absence (NH) or presence (E2) of 10−6 M 17β-estradiol. Bound proteins were eluted and separated on SDS–10% polyacrylamide gels. Labelled proteins were detected by fluorography. The input lane represents 20% of the total volume of the lysate used in each reaction.