Abstract
The African buffalo (Syncerus caffer) is an iconic species of South African megafauna. As the farmed buffalo population expands, the potential impacts on population health and disease transmission warrant investigation. A retrospective study of skin biopsy and necropsy samples from 429 animals was performed to assess the spectrum of conditions seen in buffaloes in South Africa. Determination of the cause of death (or euthanasia) could not be made in 33.1% (136/411) of the necropsy cases submitted due to autolysis or the absence of significant lesions in the samples submitted. Infectious and parasitic diseases accounted for 53.5% (147/275) of adult fatal cases and non-infectious conditions accounted for 34.9% (96/275). Abortions and neonatal deaths made up 11.6% (32/275) of necropsy cases. Rift Valley fever, bovine viral diarrhoea, malignant catarrhal fever, tuberculosis, bacterial pneumonia, anaesthetic deaths, cachexia and hepatotoxic lesions were the most common causes of death. The range of infectious, parasitic and non-infectious diseases to which African buffaloes were susceptible was largely similar to diseases in domestic cattle which supports concerns regarding disease transmission between the two species. The similarity between diseases experienced in both species will assist wildlife veterinarians in the diagnosis and treatment of diseases in captive African buffaloes. The present study likely does not represent accurate disease prevalence data within the source population of buffaloes, and diseases such as anthrax, brucellosis and foot and mouth disease are under-represented in this study. Hepatic ductal plate abnormalities and haemorrhagic septicaemia have not, to our knowledge, been previously reported in African buffaloes.
Keywords: African buffalo, Syncerus caffer, disease, pathology, wildlife veterinarians
Introduction
African buffaloes (Syncerus caffer) are one of the most iconic species of South African megafauna and are included among the ‘Big Five’, animals prized by big-game hunters and ecotourists alike. As such, in recent decades there has been significant growth in the South African game farming industry in order to meet the increasing demand for buffaloes to stock game reserves and wildlife parks. Current population estimates range from 45 000 to 100 000 farmed buffaloes with an additional 40 000–50 000 free-ranging buffaloes within the Greater Kruger National Park Complex (KNP), Hluhluwe/iMfolosi Park (HiP) and Addo National Park (ANP, personal communication, J. Dippenaar).
As the farmed buffalo population expands, the potential impacts on population health and disease transmission are important. Standard farming practices may affect disease epidemiology via artificially increased population densities, intensive nutritional management, and increased contact between buffaloes, domestic cattle, and the human population. One example that highlights the potential for disease transmission is the outbreak of bovine tuberculosis (Mycobacterium bovis) in the free-ranging buffalo population in KNP. First diagnosed in KNP buffaloes in 1990 (Bengis et al. 1996), by 1998 the prevalence of tuberculosis in buffaloes had risen to 38.2% in the southernmost region of the park (De Vos et al. 2001; Rodwell et al. 2001). Bovine tuberculosis is now considered endemic within KNP and HiP, with infection documented in many other South African wildlife species, including a variety of ruminants, carnivores and primates (Michel et al. 2006). As members of the family Bovidae, African buffaloes are susceptible to many of the same economically important infectious diseases as domestic cattle (Caron et al. 2016; Michel & Bengis 2012). Most of the published literature on diseases in buffaloes therefore describe the epidemiology and seroprevalence of infectious diseases relevant to cattle including foot and mouth disease (Thomson & Bastos 2004; Wekesa et al. 2015); arboviruses (Kading et al. 2013); Rift Valley fever (Beechler et al. 2015; Jori et al. 2015); lumpy skin disease (Fagbo, Coetzer & Venter 2014); bovine viral diarrhoea virus (BVDV) (Kabongo & Van Vuuren 2004); malignant catarrhal fever (MCF) (Pfitzer et al. 2015); tuberculosis (Kalema-Zikusoka et al. 2005; Tavalire et al. 2018); non-tuberculous mycobacteria (Gcebe et al. 2013); brucellosis (Alexander et al. 2012; Gradwell et al. 1977; Herr & Marshall 1981; Motsi et al. 2013; Tanner et al. 2014); anthrax (Cossaboom et al. 2019; De Vos & Turnbull 2004; Ebedes 1976); leptospirosis (Atherstone, Picozzi & Kalema-Zikusoka 2014); Q-fever (Ndeereh et al. 2017) and theileriosis (Chaisi et al. 2011). Parasite surveys and case reports are also recorded including infestations with coccidia (Gorsich et al. 2014); giardia (Hogan et al. 2014); ixodid ticks (Moyo, Chakuya & Sungirai 2018); haemoparasites (Eygelaar et al. 2015; Gonçalves et al. 2018; Sisson et al. 2017); nematodes (Taylor, Skinner & Boomker 2013); sarcocysts (Dubey et al. 2014; Quant et al. 1997); sarcoptes (Munang’andu et al. 2010); demodex (Dräger & Paine 1980; Wolhuter et al. 2009); schistosomes (Beechler et al. 2017); taenia (Muma et al. 2014); and thelazia (Ayebazibwe et al. 2010). Very little information is present on non-infectious conditions (Lawrence, Foggin & Prozesky 2010). Vigilant disease surveillance and studies on the interface between such diseases in cattle and buffaloes are invaluable in protecting both animal and public health as well as food security (Ritz et al. 2013). If current and future disease surveillance programmes are to be successful, it is first necessary to fully understand the range of diseases to which buffaloes are susceptible. For these reasons, a retrospective study was performed to assess the spectrum of conditions seen in African buffaloes in South Africa, and to provide a frame of reference for veterinarians, pathologists, wildlife biologists, and managers of game reserves.
Materials and methods
A retrospective review of buffalo cases submitted for pathological evaluation to four South African laboratories between 2001 and 2015 was performed. Gross necropsy and histopathology reports, as well as archival histopathology slides, were provided by the National Zoological Garden (NZG) (South African National Biodiversity Institute), the Faculty of Veterinary Science (FVS) at the University of Pretoria, Vet Diagnostix, and IDEXX Laboratories.
Each case was reviewed by a single pathologist (DW). Clinical history, sex, age, date of post-mortem examination, body condition, pathological findings and the results of ancillary tests were recorded where available. Estimated age was supplied by submitting veterinarians. All haematoxylin and eosin (H&E) stained slides as well as any accompanying special stains and immunohistochemical stains were reviewed, and morphologic diagnoses were assigned for each organ system. Each case was also assigned a final diagnosis to highlight the major disease process and cause of death or reason for euthanasia.
Ethical considerations
This article followed all ethical standards for research without direct contact with human or animal subjects.
Results
In total, archival materials from 429 individual African buffaloes were available for review. In 334 animals, sex was provided on submission: 178 (53.3%) females and 156 (46.7%) males. In 362 cases, an estimated age was provided: 190 (52.5%) adults, 51 (14.1%) subadults, 72 (19.9%) juveniles, 32 (8.8%) neonates and 17 (4.7%) foetuses. Many more animals were recorded as coming from game farms (n = 149, 50.3%) and national parks (n = 131, 44.3%) than from zoos (n = 16, 5.4%). As many cases had no information available regarding sex (n = 95, 22.1%), age (n = 67, 15.6%) and/or captivity status (n = 133, 31.0%), these characteristics were excluded from further analysis.
Biopsy cases
Skin biopsies were submitted from 18 (6.1%, 18/429) buffaloes, two of which showed non-specific lymphoplasmacytic perivascular dermatitis with no identifiable cause. Intralesional bacteria, primarily cocci, were present in three cases with ulcerative and suppurative dermatitis. Skin biopsies from three buffaloes had segmental epidermal hyperplasia with associated parakeratosis, adnexal structure atrophy, and mild pigmentary incontinence with minimal associated dermal inflammation. Three cases of cutaneous fibropapilloma were characterised by exophytic masses composed of multiple frond-like projections lined by hyperplastic keratinised stratified squamous epithelium with variable orthokeratosis and parakeratosis. Epithelium was supported by dermal projections of densely collagenous fibrovascular stroma with many reactive fibroblasts. Demodex infestation was associated in proliferative, hyperkeratotic and granulomatous dermatitis (n = 1); while infestation with Psoroptes spp. was associated with proliferative and ulcerative dermatitis (n = 2). One lipid granuloma; two cases of focal necrotising ulcerative bacterial vulvar dermatitis with coccoid bacteria on the skin surface; one suspected endocrine dermatosis; and one case of leukoderma were diagnosed.
Determination of the cause of death (or euthanasia) could not be made in a large portion of the necropsy cases submitted (33.1%, 136/411). Failure to make a diagnosis was because of autolysis (6.3%, n = 26) or there being no life-threatening lesions in the tissues submitted (26.8%, n = 110). The cause of death for the remaining fatal cases (n = 275, 66.9%) is listed in Tables 1–4. Cause of death was based on the presence of life-threatening lesion/s in the tissues submitted, supported by ancillary clinical and microbiological data, where available. Where relevant, concurrent significant lesions are also reported.
TABLE 1.
Cause of death in 275 African buffaloes: Abortion and neonatal death.
| Category | Cause of death | Number | Proportion (%) |
|---|---|---|---|
| Abortion | 19 | 6.9 | |
| No cause determined | 8 | 2.9 | |
| Rift Valley fever virus infection | 8 | 2.9 | |
| Bovine viral diarrhoea virus infection | 2 | 0.7 | |
| Staphylococcus lentus infection | 1 | 0.4 | |
| Neonatal death | 13 | 4.7 | |
| Congenital biliary atresia | 5 | 1.8 | |
| Dystocia | 2 | 0.7 | |
| Bovine viral diarrhoea virus infection | 1 | 0.4 | |
| Congenital hydrocephalus | 1 | 0.4 | |
| Traumatic injury | 1 | 0.4 | |
| Omphalophlebitis | 1 | 0.4 | |
| Septicaemia | 1 | 0.4 | |
| Nutritional myopathy | 1 | 0.4 | |
|
| |||
| Total | 32 | 11.6 | |
TABLE 4.
Miscellaneous non-fatal lesions in African buffaloes.
| Cause of death | Number | Proportion (%) |
|---|---|---|
| Anaesthetic related | 20 | 4.7 |
| Cachexia | 19 | 4.4 |
| Hepatotoxicity | 11 | 2.6 |
| Capture myopathy | 9 | 2.1 |
| Myocardial necrosis | 7 | 1.6 |
| Rumen acidosis | 7 | 1.6 |
| Trauma | 5 | 1.2 |
| Aspiration pneumonia | 2 | 0.5 |
| Salt and microcystin toxicity | 2 | 0.5 |
| Subacute meningoencephalitis | 2 | 0.5 |
| Oesophageal phytobezoar | 1 | 0.2 |
| Clostantel toxicity | 1 | 0.2 |
| Drowning | 1 | 0.2 |
| End stage kidney disease | 1 | 0.2 |
| Haemorrhagic enteritis | 1 | 0.2 |
| Pressure myopathy | 1 | 0.2 |
| Tick toxicosis | 1 | 0.2 |
| Multicentric lymphoma | 1 | 0.2 |
| Acute nephrosis | 1 | 0.2 |
| Cerebrocortical laminar necrosis | 1 | 0.2 |
| Pregnancy toxaemia | 1 | 0.2 |
| Acute liver necrosis | 1 | 0.2 |
Abortions and neonatal deaths
Abortions and neonatal deaths accounted for 11.6% (32/275) of fatal cases. The number and proportion of cases of abortion or neonatal death are shown in Table 1.
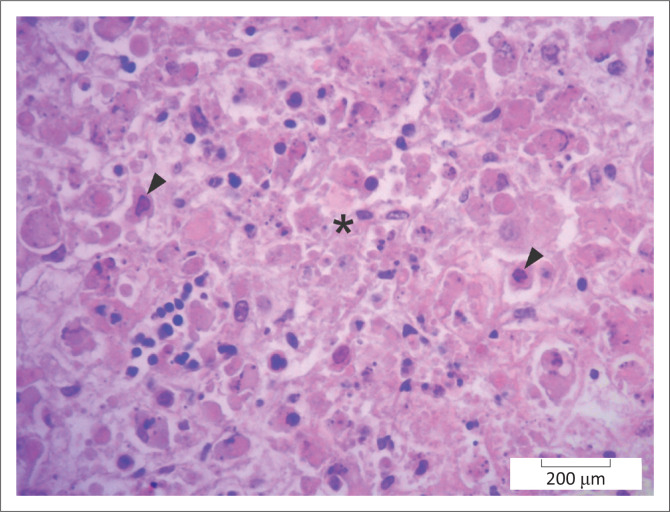
Of the total 32 cases, 19 (6.9%) cases were abortions. The cause of the abortions was not established in eight cases. Eight foetuses had lesions consistent with Rift valley fever (RVF) virus infection: severe multifocal random to massive necrotising hepatitis with (n = 7) or without (n = 1) intranuclear immunohistochemically positive viral inclusions (Figure 1). One case was positive on a polymerase chain reaction (PCR) test. Tissues from two foetuses tested positive on immunohistochemistry for BVDV and had cerebellar hypoplasia as well as lymph node and/or thymic lymphoid atrophy or hypoplasia. Staphylocococcus lentus was isolated from one case with intravascular cocci in the lung and kidney but no other lesions.
FIGURE 1.
Liver, African buffalo. Area of hepatocellular necrosis (*) because of Phlebovirus infection (Rift Valley Fever). Several necrotic hepatocytes have intranuclear viral inclusions (arrowheads). Haematoxylin and eosin.
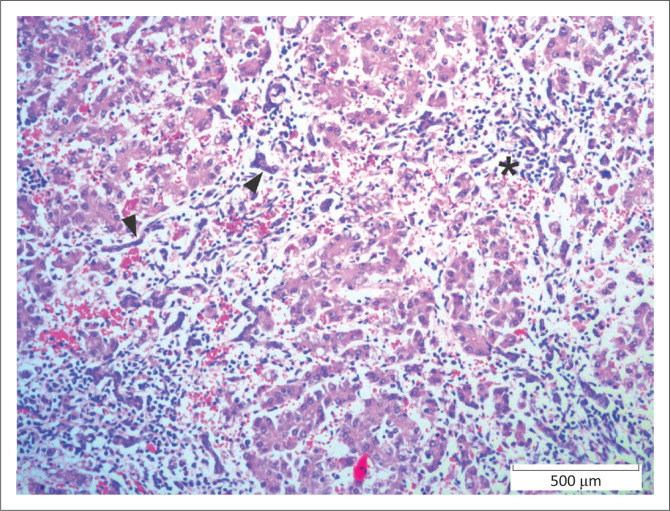
Of the 13 (4.7%) neonates submitted, five had congenital hepatic ductal plate malformation, one of which had concurrent bronchiolar dysplasia with associated pulmonary alveolar atelectasis and acute neutrophilic interstitial pneumonia; and another had concurrent pneumonia because of aspiration of milk. In these cases, there was marked variation in lobular size and poor organisation of hepatic cords. Portal triads had numerous small tortuous bile duct profiles that frequently extended into surrounding parenchyma and spanned adjacent portal areas and central veins (see Figure 2). Associated inflammation and fibrosis were minimal.
FIGURE 2.
Liver, African buffalo. Hepatic ductal plate abnormality present at birth. Note the periportal lymphoplasmacytic inflammation (*) and multiple undeveloped bile ductules (arrowheads). Haematoxylin and eosin.
The death of the remaining neonates was attributed to dystocia (n = 2), BVDV infection (n = 1), congenital hydrocephalus (n = 1), traumatic injury (n = 1); necropurulent pleuritis and cystitis because of omphalophlebitis (n = 1); multifocal fibrinonecrotic hepatitis attributed to septicaemia (n = 1); and polyphasic myocardial and skeletal muscle necrosis suggestive of nutritional myopathy (n = 1).
Infectious and parasitic diseases
Infectious and parasitic diseases accounted for 53.5% (147/275) of fatal cases. Table 2 shows the number and proportion of buffaloes that died because of infectious or parasitic diseases.
TABLE 2.
Cause of death in 275 African buffaloes: Infectious and parasitic diseases.
| Category | Cause of death | Number | Proportion (%) |
|---|---|---|---|
| Bacterial | 106 | 38.5 | |
| Bovine tuberculosis | 55 | 20.0 | |
| Bacterial pneumonia | 13 | 4.7 | |
| Clostridial infections | 9 | 3.3 | |
| Septicaemia | 8 | 2.9 | |
| Bacterial enterocolitis | 7 | 2.5 | |
| Abscesses | 6 | 2.2 | |
| Haemorrhagic septicaemia | 6 | 2.2 | |
| Traumatic thoracic infection | 1 | 0.4 | |
| Bacterial peritonitis | 1 | 0.4 | |
| Viral | 26 | 9.5 | |
| Malignant catarrhal fever | 15 | 3.5 | |
| Viral encephalitis | 4 | 1.5 | |
| Viral enteritis | 4 | 1.5 | |
| Viral pneumonia | 2 | 0.7 | |
| Rabies | 1 | 0.4 | |
| Fungal | 3 | 1.1 | |
| Rumenitis | 2 | 0.7 | |
| Abomasitis | 1 | 0.4 | |
| Parasitic | 12 | 4.4 | |
| Theileriosis | 7 | 2.5 | |
| Coccidiosis | 4 | 1.5 | |
| Myocardial cestodiasis | 1 | 0.4 | |
|
| |||
| Total | 147 | 53.5 | |
Bacterial
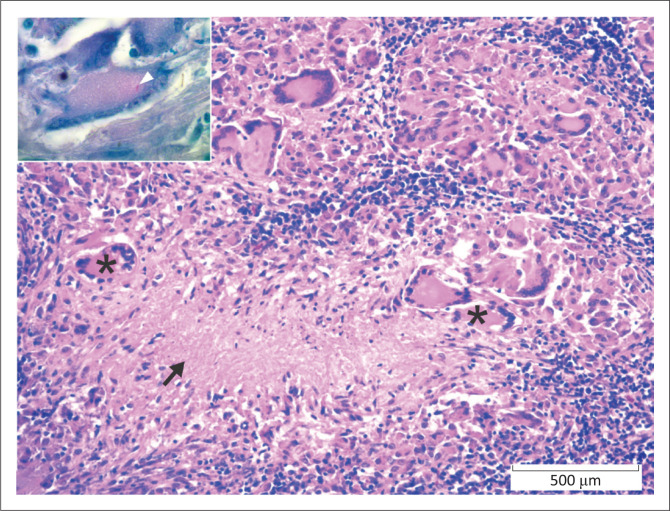
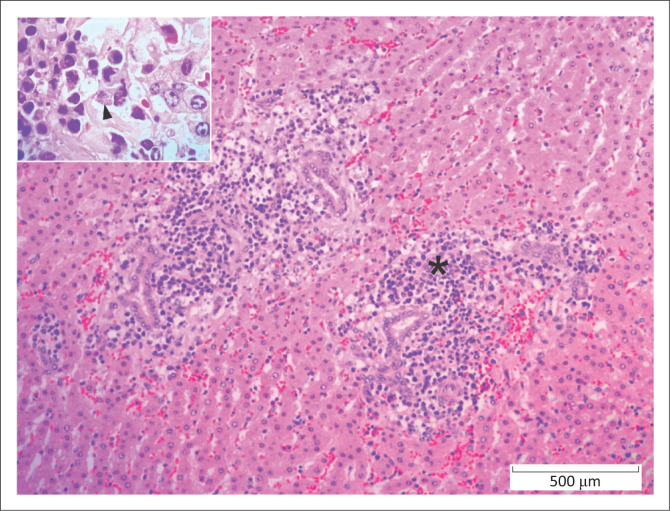
Bacterial conditions accounted for 38.5% (106/275) of fatal cases. The most common bacterial disease was tuberculosis, characteristic lesions of which were identified and detected in lymph nodes, lung and, rarely, in other tissues in 55 (20.0%) buffaloes. Lesions consisted of granulomas with multinucleated giant cells, central necrosis, peripheral lymphoplasmacytic inflammation, and in some cases mineralisation (Figure 3). Nineteen cases had small numbers of intra-histiocytic acid-fast bacilli morphologically compatible with Mycobacterium sp. (Ziehl Neelsen stain). In five of these cases, M. bovis infection was confirmed via PCR test. Scattered macrophage clusters in lung or lymph node were interpreted as early lesions in 17 cases.
FIGURE 3.
Lymph node, African buffalo. Granuloma with multinucleated giant cells (*) and central necrosis (arrow). Haematoxylin and eosin. Inset: A rare intracytoplasmic acid-fast (Ziehl-Neelsen) bacillus (arrowhead) is present within a giant cell. Ziehl Neelsen.
Bacterial pneumonia and/or pleuropneumonia, with or without sepsis, was responsible for death in 13 (4.7%) adult buffaloes. Mannheimia haemolytica was isolated from one case. Marked multifocal subacute fibrinosuppurative to necrotising bronchopneumonia with unidentified coccobacilli was seen in three animals, one of which had concurrent theileriosis. One case had concurrent viral enteritis.
Confirmed or suspected clostridial infections were seen in nine (3.3%) buffaloes. Four buffaloes had necrotising suppurative myositis with variable numbers of plump spore-firming bacilli. Myositis was confirmed to be because of C. septicum (n = 1) and C. chauvoei (n = 1) by immunofluorescent antibody test; in two additional cases no further testing was performed. In five animals, lesions compatible with Clostridium spp. infection were seen: mild multifocal acute necrotising myocarditis with thrombosis (n = 1); necrohaemorrhagic enteritis (n = 1); and severe necrohaemorrhagic abomasitis, with intra-lesional large bacterial rods (n = 3). Cerebellum Purkinje cell necrosis, consistent with intoxication with Solanum kwebense, was also present in one of the latter cases.
In eight (2.9%) buffaloes, death was judged to be because of septicaemia, without identification of the pathogens responsible. Lesions included moderate multifocal random acute necrotising lesions in the liver (n = 4), lung (n = 3), spleen (n = 2), hepatic portal triads (n = 1), pleura (n = 1), myocardium and heart valves (n = 1). In one case, septicaemia was associated with severe chronic fibrinosuppurative pleuritis with intralesional coccobacilli (associated with a penetrating foreign body from the reticulum); in another the source of the septicaemia was valvular endocarditis.
Seven (2.5%) buffaloes died of complications of confirmed or suspected bacterial enterocolitis, in which there was necrosis and ulceration of the intestinal mucosa accompanied by predominately fibrinopurulent inflammation. From one animal with concurrent peritonitis, Escherichia coli was isolated; in another a Salmonella spp. was cultured. Samples were not submitted for culture in other animals. Mild segmental acute necrotising suppurative enteritis had intralesional coccobacilli (n = 1); severe multifocal acute ulcerative enteritis with mixed bacteria (n = 1); and mild focal acute ulcerative colitis with intralesional bacilli (n = 1) were reported. Bacterial disease was suspected because of mild diffuse acute neutrophilic enteritis in one animal; and to haemorrhagic enteritis and jejunal volvulus in another.
In six (2.2%) buffaloes, death was attributed to the presence of abscesses. One of two buffaloes with brain abscesses also had rumen acidosis. Trueperella pyogenes was isolated (n = 1) and intralesional filamentous bacteria consistent with Fusobacterium necrophorum (n = 1) were present in buffaloes with abscesses in multiple tissues. One animal each had T. pyogenes abscesses in the mandible and renal pelvis.
Five (1.8%) buffaloes died because of haemorrhagic septicaemia caused by Pasteurella multocida. Affected animals had variable combinations of multifocal necrohaemorrhagic or fibrinopurulent bronchopneumonia and pleuritis (n = 2); pulmonary haemorrhage (n = 1), epicarditis (n = 1), hepatitis (n = 1), lymphadenitis (n = 3), tonsillitis (n = 2), lymphangitis (n = 1), nephritis (n = 1), myositis (n = 1), thymitis (n = 1) and adrenocortical haemorrhage (n = 1) with small numbers of intravascular and/or intralesional coccobacilli. One additional buffalo with severe multifocal to coalescing subacute necropurulent pharyngeal myositis was presumed to also have had haemorrhagic septicaemia.
One buffalo died of severe chronic fibrinosuppurative epicarditis attributed to traumatic inoculation of bacteria as a result of foreign body migration from the reticulum. One animal died as a result of marked acute fibrinosuppurative peritonitis with intralesional coccobacilli.
Viral
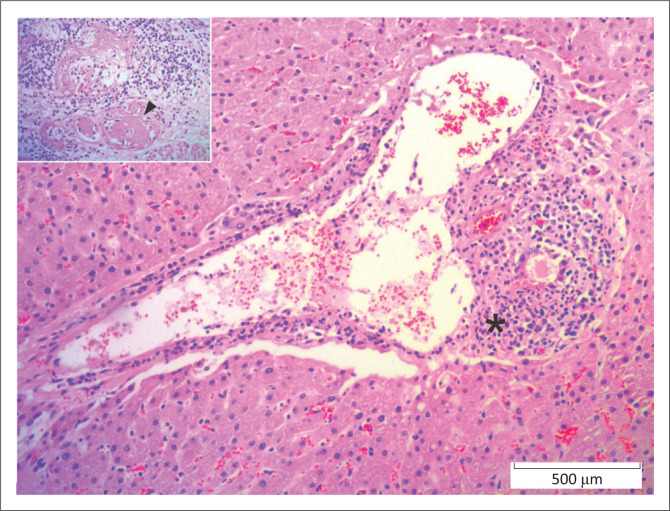
Viral diseases accounted for 9.5% (26/275) of fatal cases. Fifteen individuals (3.5%) had lesions consistent with MCF. Necrotising vasculitis with fibrinoid change and associated mural and perivascular lymphoplasmacytic inflammation were most common in the liver (n = 10), kidney (n = 9) and lung (n = 9), but were also seen in the intestine (n = 5), heart (n = 5), spleen (n = 3), lymph node (n = 2), rumen (n = 2), brain (n = 2) and retrobulbar adipose tissue (n = 1) (Figure 4). In seven of these cases, ancillary PCR testing performed at the time of necropsy confirmed Ovine Herpes Virus 2 (OvHV-2, n = 3) or Alcelaphine Herpesvirus-1 (AlHV-1, n = 4)
FIGURE 4.
Liver, African buffalo. Portal triad with necrotising and lymphocytic vasculitis (*) because of Ovine herpes virus-2 infection (malignant catarrhal fever). Inset: vascular profiles are obscured by fibrinoid necrosis (arrowhead). Haematoxylin and eosin.
Viral encephalitis was diagnosed in four (1.5%) buffaloes as a result of a history of ataxia, recumbency and acute death and the presence of spinal cord glial apoptosis, neuronal degeneration and/or cerebral oedema. Four (1.5%) animals died of enteric lesions consistent with viral infection. Three of these buffaloes had crypt epithelial necrosis, while the remaining only had residual intestinal villus blunting and fusion, and was cachexic.
Two (0.7%) buffaloes were thought to have died of viral pneumonia because of the presence of severe multifocal to coalescing acute serofibrinous interstitial pneumonia with epithelial syncytia. However, neither virus isolation nor PCR test were performed. One affected animal also had ruminal acidosis. One animal died of confirmed rabies infection.
Fungal
Fungal diseases accounted for 1.1% (3/275) of fatal cases. Two buffaloes had proliferative and ulcerative fungal rumenitis; and in another zygomycete fungal ulcerative abomasitis resulted in perforation and peritonitis.
Parasitic
Parasitic diseases accounted for 4.4% (12/275) of fatal cases. Theileriosis (Figure 5) was diagnosed in seven (2.5%) buffaloes with widespread lymphoplasmacytic infiltrates, including atypical blastic cells with or without protozoa, in the hepatic portal area (n = 5), lymph node (n = 4), spleen (n = 3), renal interstitium (n = 3), myocardium (n = 2) and/or pulmonary interstitium (n = 1). Only one case was confirmed by PCR test. Additional lesions included marked subacute fibrinosuppurative bronchopneumonia with few fibrin thrombi (n = 1), marked segmental acute fibrinonecrotic and ulcerative abomasitis (n = 1), marked focal subacute necrotising nephritis (n = 1) and severe subacute fibrinonecrotic lymphadenitis (n = 1).
FIGURE 5.
Liver, African buffalo. Lymphoplasmacytic portal hepatitis (*) because of Theileriosis infection. Inset. Lymphoblastic cell with intracytoplasmic schizonts (arrowhead). Haematoxylin and eosin.
Four (1.5%) buffaloes died of severe infestation of the intestine and diarrhoea associated with coccidiosis. Coccidia was most commonly present in the small intestine, and associated with mild often eosinophilic inflammation. Coccidian burden varied considerably between individuals and between different intestinal sections of the same individual. In addition, mild intestinal epithelial infestation with coccidia was present in 10 (3.6%) other animals. No other significant lesions in the tissues submitted were present in two of these cases; and one animal each had concurrent compressive myelopathy, displaced abomasum, end stage kidney disease, haemochromatosis, pneumonia as a result of aspiration of rumen fluid and cachexia.
Multiple encysted larval cestodes were associated with mild granulomatous myocarditis in one buffalo.
Non-infectious causes of death
Non-infectious conditions accounted for 34.8% (96/276) of fatal cases. The number and proportion of non-infectious causes of death is listed in Table 3. Death during or shortly after anaesthesia was seen in 20 (7.2%) buffaloes. Lesions seen included acute renal tubular necrosis (n = 4), pulmonary congestion and/or oedema (n = 4), myocardial oedema (n = 1), pulmonary atelectasis (n = 1), intact erythrocytes in lymph node sinuses (interpreted as drainage from areas of haemorrhage, n = 1), acute myocardial necrosis (n = 1), perivascular cerebral haemorrhage (n = 1), tracheal haemorrhage (n = 1), pneumonia as a result of aspiration of rumen fluid (n = 2), centrilobular liver necrosis (n = 1), focal neutrophilic necrotising haemorrhagic myocarditis (n = 1), hypoxic encephalopathy with neuronal necrosis (n = 1) and cerebral oedema (n = 1). One affected animal was cachexic with endoparasites, anaemia and sepsis. Three animals were diagnosed with anaesthetic-induced hyperthermia as a result of hepatocellular degeneration (n = 3), acute renal tubular necrosis (n = 2), multifocal fibrin thrombi (n = 1) and multifocal acute cerebral neuronal necrosis (n = 2). In four animals, no lesions were seen in the tissues submitted.
TABLE 3.
Cause of death in 275 African buffaloes: Non-infectious diseases.
| Cause of death | Number | Proportion (%) | |
|---|---|---|---|
| Anaesthetic related | 20 | 7.2 | |
| Cachexia | 19 | 6.9 | |
| Hepatotoxicity | 11 | 4.0 | |
| Myocardial necrosis | 10 | 3.6 | |
| Capture myopathy | 9 | 3.3 | |
| Trauma | 5 | 1.8 | |
| Rumen acidosis | 4 | 1.4 | |
| Aspiration pneumonia | 2 | 0.7 | |
| Salt and microcystin toxicity | 2 | 0.7 | |
| Subacute meningoencephalitis | 2 | 0.7 | |
| Oesophageal phytobezoar | 1 | 0.4 | |
| Clostantel toxicity | 1 | 0.4 | |
| Drowning | 1 | 0.4 | |
| End stage kidney disease | 1 | 0.4 | |
| Haemorrhagic enteritis | 1 | 0.4 | |
| Pressure myopathy | 1 | 0.4 | |
| Tick toxicosis | 1 | 0.4 | |
| Multicentric lymphoma | 1 | 0.4 | |
| Acute nephrosis | 1 | 0.4 | |
| Cerebrocortical laminar necrosis | 1 | 0.4 | |
| Pregnancy toxaemia | 1 | 0.4 | |
| Acute liver necrosis | 1 | 0.4 | |
|
| |||
| Total | 96 | 34.8 | |
Nineteen buffaloes (6.9%) died of malnutrition/starvation with lesions including skeletal muscle atrophy, hepatocellular atrophy with or without concomitant lipidosis, serous atrophy of peri-renal and epicardial fat, and pancreatic zymogen granule depletion. In six animals, no lesions were seen in the tissues submitted. In the remainder, the relationship between various mild lesions and death because of cachexia was uncertain: multifocal proliferative glomerulopathy; multifocal renal interstitial fibrosis; multifocal cardiomyocyte degeneration and megakaryosis, multifocal pulmonary arteriolar mineralisation, aortic arteriosclerosis, cerebral gliosis or oedema, cutaneous lice infestation, paramphistome infestation of the rumen, coccidiosis, enteritis, goitre and pneumonia as a result of aspiration of rumen fluid.
Eleven (4.0%) buffaloes died because of hepatotoxicity. Acute disease (n = 5) was characterised by centrilobular hepatocyte necrosis and loss. One affected animal also had terminal pneumonia as a result of aspiration of rumen fluid and mild acute myocardial necrosis. Chronic cases (n = 6) had additional changes including lobular collapse, hepatocellular megakaryosis, biliary hyperplasia, and bridging fibrosis. Two of these cases also had aspiration pneumonia; and one was suffering from multicentric lymphoma. Similar lesions were seen in an additional animal that was also cachexic. Given the paucity of historical information for most cases, a specific cause could not be determined for any case.
Ten (3.6%) buffaloes died as a result of multiphasic subacute to chronic multifocal myocardial necrosis with or without myofibre atrophy (n = 3), myoglobinuria (n = 1) and chronic pulmonary oedema (n = 1). These lesions were interpreted as nutritional or toxic myopathy. One had fibrinoid vascular necrosis attributed to concurrent copper deficiency although no tissue mineral testing was performed. Myocardial fibrosis in one buffalo was interpreted as chronic capture or nutritional myopathy. Two animals had concurrent marked diffuse epidermal hyperplasia and ortho/parakeratosis with secondary bacterial and fungal dermatitis in addition to the myocardial lesions.
Nine (3.3%) animals were diagnosed with capture myopathy because of history of recent anaesthesia and/or translocation and the presence of acute skeletal muscle degeneration and/or necrosis. Additional lesions seen in these cases included acute myocardial necrosis (n = 5), myoglobinuric nephrosis (n = 2), centrilobular hepatic necrosis (n = 2) and pulmonary congestion and oedema (n = 1). One animal had acute myocardial necrosis and subacute skeletal muscle necrosis. One affected animal also had lesions consistent with MCF.
Five (1.8%) animals died or were euthanised as a result of traumatic injury: a fractured foot, gangrenous wound, and two by predation. In one case, the trauma was not specified.
Four (1.4%) buffaloes died of rumen acidosis segmental ruminal epithelial hyperplasia and parakeratosis, with multifocal areas of epithelial necrosis, microabscesses and ulceration and/or mucosal oedema. One also had ruminal infestation with paramphistomes; and another with suspected viral pneumonia. Two animals with ruminal ulceration or rumenitis also had secondary embolic pneumonia. An additional four animals had similar mild ruminal lesions, but died of other causes: brain abscess (n = 1), haemorrhagic septicaemia (n = 1), anaesthetic death (n = 1), cachexia (n = 1),
Two (0.7%) animals died of aspiration pneumonia as a result of aspiration of activated charcoal (n = 1) and a foreign body (n = 1), respectively. Two (0.7%) died of cerebral oedema, acute laminar cortical necrosis and cerebral perivascular haemorrhage; as well as periacinar hepatocyte necrosis and skeletal muscle necrosis. The diagnosis was a combination of water deprivation and blue-green algal toxicity.
Two buffaloes (0.7%) died of moderate multifocal lymphoplasmacytic meningoencephalitis of unknown aetiology. One of these animals had significant numbers of macrophages in the inflammatory response; in the other, inflammation was necrotising and concurrent pneumonia as a result of aspiration of rumen fluid was present.
The following single cases of death or euthanasia were seen: because of choke, bloat and pulmonary congestion and oedema as a result of oesophageal phytobezoar; presumed closantel toxicity with marked cerebral white matter spongiosis, mild neuronal degeneration and gliosis, perivascular cerebral oedema and skeletal muscle degeneration; drowning; a combination of end stage kidney disease and haemochromatosis; pressure myopathy; rhabdomyolysis attributed to tick toxicosis; multicentric small cell lymphoma; acute nephrosis the cause of which could not be determined; marked acute cerebrocortical laminar necrosis; pregnancy toxaemia; acute liver necrosis of unknown cause.
Miscellaneous non-fatal lesions, not the cause of death
In addition to the cause of death in few cases miscellaneous lesions were also present. These were however deemed of no specific clinical significance. The number and proportion of cases with miscellaneous lesions are listed in Table 4. The proportion of cases is based on the number of animals for which the relevant tissue was submitted.
Lymph node reactive change was noted in 154 (62.6%, 154/202) individuals for which lymph nodes were submitted: lymphoid follicular hyperplasia, sinus histiocytosis and/or plasmacytosis, and mild lymph node oedema. In 35 (14.2%) cases, lymph node changes consistent with draining areas of haemorrhage were present, including flooding of medullary sinuses with erythrocytes and proteinaceous fluid. Additionally, there were variable numbers of sinus histiocytes with intracytoplasmic erythrocyte fragments and/or dark brown granular hemosiderin pigment. Eight (3.3%) adult individuals had lymphoid depletion noted in the lymph nodes with a paucity of lymphocytes and follicular structures. One animal had pyogranulomatous lymphadenitis with intralesional bacilli (not consistent with tuberculosis); and another buffalo had generalised neutrophilic lymphadenitis of which a cause could not be determined.
In addition, 44 (18.0%, 44/244) buffaloes had varying degrees of splenic lymphoid follicular hyperplasia and sinus histiocytosis and/or plasmacytosis consistent with reactive change. Reactive change was considered an incidental finding resulting from antigenic stimulation because of a variety of causes. In 43 (17.6%) individuals, the splenic red pulp contained increased numbers of hemosiderin-laden macrophages. Six (2.5%) adult animals had splenic lymphoid atrophy, in which there were both decreased numbers of lymphoid follicles and a paucity of lymphocytes within follicles.
Fourteen (60.9%, 14/23) animals had ruminal epithelial mineralisation. Superficial ruminal epithelial cells were deeply basophilic, stippled, and fragmented. In some cases, mineralisation was accompanied by mild epithelial hyperplasia. In two (8.7%, 2/23) cases, the rumen was infested with paramphistomes.
One animal had focal neutrophilic and eosinophilic abomasitis, which was likely as a result of internal parasites. Enterocolitis was present in 14 (27.5%, 14/51) buffaloes with no apparent specific aetiology. Increased numbers of lymphocytes, plasma cells, and eosinophils in varying proportions infiltrated the lamina propria of the small and/or large intestines with minimal mucosal damage.
The most prevalent hepatic lesion, noted in 42 (15.0%, 42/280) animals, was mild lymphoplasmacytic periportal hepatitis with mild biliary hyperplasia and periportal fibrosis. Hepatic haemochromatosis was present in eight (2.9%) animals. One buffalo had hepatic infestation with Fasciola spp.
Thirty two (10.4%, 32/309) buffaloes had small perivascular and peri-bronchial clusters of macrophages with abundant dark brown to black, granular, sometimes refractile, intracytoplasmic pigment. In some individuals, similar pigment-laden macrophages were also noted in the medullae of tracheobronchial lymph nodes. Pigment was consistent with carbon and/or silica and accumulation within macrophages was likely secondary to chronic inhalation of particulate matter (anthracosis). One animal each had mild eosinophilic pneumonia; and mild lymphohistiocytic pneumonia.
Twenty five (9.5%, 25/264) buffaloes had mild chronic interstitial nephritis, characterised by multifocal interstitial infiltrates of lymphocytes, plasma cells, occasional macrophages, and variable amounts of accompanying fibrosis. Eleven (4.2%) animals had small numbers of renal tubules, predominately within the medulla, with mineralised foci in their lumina. Seven (2.7%) individuals had membranous glomerulonephritis characterised by mild to moderate thickening of capillary basement membranes and Bowman’s capsules as well as segmental to global increases in the amount of mesangial matrix.
Ten (71.4%, 10/14) skin samples from necropsy cases had dermal inflammation but no detectable etiologic agents. Inflammation ranged from neutrophilic to lymphoplasmacytic, and in one case granulomatous, and there were variable degrees of associated dermal fibrosis and epidermal hyperplasia.
Six (2.3%, 6/265) individuals had small focal to multifocal areas of myocardial and/or endocardial mineralisation that were likely related to previous resolved injury. One animal had mild localised lymphocytic and neutrophilic vasculitis.
Five (12.3%, 5/38) animals had adrenocortical hyperplasia. Four buffaloes (16%, 4/25) had mild hyperplasia of the thyroid gland (hyperplastic goitre). Two (2%, 2/100) had skeletal muscle cysts compatible with Sarcocystis cafferi in skeletal muscle (adjacent to the trachea). One animal each had cerebral oedema; and spinal cord axonal degeneration. No lesions were noted in the reproductive system in the 14 cases for which reproductive tissue was available.
Discussion
Based on these findings, the range of infectious, parasitic and non-infectious diseases to which African buffaloes are susceptible is largely similar to diseases in domestic cattle which supports concerns regarding disease transmission between the two species. The similarity between diseases experienced in both species may assist wildlife veterinarians in the diagnosis and treatment of diseases in captive African buffaloes. The histological appearance of Mannheimia haemolytica associated pneumonia, Clostridium myositis and abomasitis, E. coli and Salmonella enteritis, Trueperella pyogenes and Fusobacterium necrophorum infections, rabies were similar to those described in cattle (eds. Coetzer, Thomson & Tustin 2004).
In addition to those diseases known to be transmitted from buffaloes to cattle, some diseases were likely transmitted to buffaloes from cattle. The presence of BVD in buffalo foetuses likely reflects infection from cattle, as the virus has not been detected in buffaloes from the KNP (Kabongo & Van Vuuren 2004). Similarly, skin lesions because of bovine papilloma virus, as seen in these buffaloes, are recorded in several wild ruminants, including buffaloes (Williams et al. 2011). However, testing for viral genetic material was not performed so this diagnosis could not be confirmed.
While the source of viral encephalitis was not determined in the buffaloes in this series, the lesions resembled those in domestic horses in South Africa as a result of West Nile virus infection (Williams 2014). Serological evidence of alpha and flavivirus infections is recorded in various African wild animals (Kading et al. 2013). Many species of domestic and wild animals may therefore be susceptible to disease because of these infections, particularly when climate conditions favour the proliferation of insect vectors and/or host immune suppression (e.g. late winter especially during periods of drought). Similarly, the presence of buffalo abortions because of RVF, as well as cases of infestation with Fasciola spp. and paramphistomes, are suggestive that both cattle and buffaloes may be affected by diseases from a common source. Cases of MCF were similar to those described previously (Pfitzer et al. 2015). The OHV-2 infection likely indicate the infection of buffaloes from domestic ruminants; while cases because of AlHV-1 emphasise the need to manage contact between wild ruminant species on game farms.
The appearance of tuberculous lesions and those of theileriosis corresponded to previous reports in buffaloes (Bengis et al. 1996; Clift et al. 2020; De Vos et al. 2001; Laisse et al. 2011). Sarcocyst morphology was consistent with Sarcocystis cafferi as previously reported in African buffaloes (Dubey et al. 2014; Quant et al. 1997) and occurred only in free-ranging buffaloes presumably because, in such animals, predators are present to complete the indirect life cycle.
Some cattle diseases reported here have not, to our knowledge, been previously reported in African buffaloes. Hepatic ductal plate abnormalities are uncommon in cattle and typically associated with more fibrosis than was present in these cases (Bourque et al. 2001; Yoshikawa et al. 2002). A genetic predisposition is more likely than a toxic aetiology, as toxic hepatic ductal plate abnormalities are not seen in other ruminants in South Africa. Haemorrhagic septicaemia, with similar pathology to that described in African buffaloes in this study, is common in water buffaloes (Bubalis bubalis) (Praveena et al. 2014) suggesting that Bovidae share a predisposition to Pasteurella multocida infection. Staphylococcus lentus is part of a coagulase negative antibiotic resistant Staphylococcus sciuri group that is widespread in nature, and principally causes wound infections and mastitis in animals (Stepanovic et al. 2001). Its role in the abortion of the single buffalo in this series is uncertain.
Given the importance of infectious disease in buffaloes, it is not surprising that non-infectious disease is less frequently reported. The pathology of deaths that occur during or shortly after anaesthesia has not, to our knowledge, been documented in African buffaloes. Cardiorespiratory, renal and central nervous system changes were similar to those described in wildlife in general (Meltzer & Kock 2012). Similarly, myocardial and skeletal muscle lesions were characteristic of capture myopathy (Breed et al. 2019). The presence of subacute muscle lesions in animals dying with acute capture myopathy could be because of prior immobilisation or concurrent nutritional myopathy. Hepatic haemochromatosis is rare in domestic cattle, although cases are described in the Saler breed (O’Toole et al. 2001). The cause in buffaloes is unknown.
Various conditions, often seen in cattle being intensively managed, were also seen in buffaloes. Coccidian speciation was not performed, though Eimeria sp. infection has previously been reported in buffaloes. The prevalence and intensity of coccidian infestation in free-ranging buffaloes is affected by season (Gorsich et al. 2014). Wildlife veterinarians may need to examine factors such as animal density, water drainage into and faecal contamination of feeding areas in farmed buffaloes, which may facilitate occasional fatal coccidiosis.
Some conditions likely had a nutritional aetiology, as a result of artificial nutrition and/or drought conditions. Lesions consistent with acidosis likely explain the cases of fungal rumenitis and brain abscesses. Given the presence of lesions characteristic of nutritional myopathy, and zinc-responsive dermatosis, supplementation of multiple minerals should be considered, particularly if buffaloes are fed irrigated lucerne (Mbatha et al. 2012). The presence of nutritional myopathy in a neonate is suggestive of significant underlying nutritional deficiency disease in adult buffalo cows. Since free-ranging buffaloes do not normally suffer from blue-green algae toxicity (Bengis et al. 2016) because of their habit of wading into water bodies to drink, the water deprivation and blue-green algae toxicity cases were likely because of accidental temporary suspension and poor hygiene of water supply in farmed animals.
In addition, myocardial (Lawrence et al. 2010) as well as hepatic and neurological lesions characteristic of plant toxicities in cattle in South Africa (Kellerman et al. 2005) indicate that, as in cattle, buffaloes with insufficient alternative good quality feed may ingest toxic plants. Ruminal epithelial hyperplasia and mineralisation are reported in impala (Aepyceros melampus). The lesion is seen most commonly in the dry season and at locations at lower altitude where forage protein is low and dietary fibre and browse content is high, and may therefore be a result of a diet rich in tannins (Lane 1995). How this relates to the same lesion in buffaloes is unknown, as they are typically grazers. More research is needed on how artificial diets in captivity may affect buffalo health.
As a retrospective analysis spanning 15 years, the present study likely does not represent accurate disease prevalence data within the source population of buffaloes because of several factors. Firstly, specimens were not collected in a systematic manner. For example, reproductive tract tissue was rarely submitted, making detailed evaluation of this organ system impossible. A definitive diagnosis of abortion is most likely if foetal and placental tissues are promptly submitted to a diagnostic lab; yet placenta was rarely submitted. The proportion of abortions in which a diagnosis could be made in these buffaloes is similar to that described in cattle (Wolf-Jäckel et al. 2020).
In addition, clinical data was rarely detailed; and additional tests were often not possible because of failure to submit the relevant samples, sample post-mortem change or financial considerations. For example, had the clinical history of one bull with myoglobinuria included that it had been observed aggressively fighting with other males, the cause of muscle necrosis and subsequent pigmentary nephrosis could have been confirmed as exertional rhabdomyolysis. Similarly, without more extensive clinical history, the cause of cachexia could rarely be determined. The value of the study would have been greatly improved if such information was submitted with the tissues for analysis and a full set of tissues collected from all animals. Lastly, many tissues had significant autolytic change which hindered meaningful histological interpretation and diagnosis.
Some diseases to which buffaloes are susceptible were under-represented in this study. Diseases such as anthrax, brucellosis and foot and mouth disease (FMD) are endemic in buffaloes in KNP and other locations (Cossaboom et al. 2019; De Vos & Turnbull 2004; Gradwell et al. 1977; Herr & Marshall 1981; Motsi et al. 2013; Vosloo et al. 2009) and are diagnosed by experienced field staff without submitting samples for pathological examination. In addition, the transport of samples out of FMD endemic areas to regional laboratories is restricted. Ticks, internal parasites, haemoparasites and Bartonella spp. (Gonçalves et al. 2018) were likely similarly under-represented as blood or cytology samples were rarely submitted.
In contrast, the high prevalence of tuberculosis in this series is likely as a result of targeted surveillance for the disease in free-ranging and captive animals and the requirement to confirm the diagnosis by histological examination. Diagnosis is rarely confirmed by culture and/or PCR in free-ranging buffaloes from the endemic regions of South Africa, because of cost considerations and the ubiquitous presence of M. bovis in the buffalo population there. Farmed buffaloes that test positive for tuberculosis and are euthanised to confirm or rule out the infection for disease control purposes (Musoke et al. 2015).
This study confirmed that African buffaloes are susceptible to many of the same diseases as domestic cattle in South Africa. Therefore, in the absence of definitive tests, practitioners should assume that cattle and buffaloes share pathogens where they co-exist and that diseases in buffaloes may present similarly to those in cattle. As such, it is important to recognise that buffaloes may play a pivotal role in disease epidemiology with significant impacts on food security, public health, and wildlife conservation. For these reasons, continued and diligent surveillance of captive, farmed, and free-ranging populations alike is imperative.
Acknowledgements
The authors thank Profs A. Kotze and K. Terio, respectively, for providing the platform for the collaboration. This study would not have been possible without the wildlife farmers and veterinarians who gave permission for the samples to be used for this research. Small numbers (1–20) of archived cases from the University of Pretoria’s Faculty of Veterinary Science (FVS), National Zoological Gardens of South Africa and Vet Diagnostix, were included in the study by pathologists Profs N. Duncan, N.P.J. Kriek, M. Williams, Drs D. Janse van Rensburg, G. van der Veen, J. Neser, J. Williams, L. Odendaal, M.L. Penrith, M.M. Steenkamp, N. O’Dell, S. Clift and J.H. Vorster. The histology laboratory of the FVS provided excellent technical assistance in processing tissues and performing routine, special and immunohistochemical staining. Drs Tiny Hlokwe, Marco Romito and Staff from the Agricultural Research Council-Onderstepoort Veterinary Institute did the polymerase chain reaction tests for tuberculosis (Dr T. Hlokwe), theileriosis (Dr M. Romito) and the immunofluorescent antibody tests for clostridial myositis (the Bacteriology Laboratory). The images were immeasurably improved by Ms E. Mayhew’s technical expertise.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors’ contributions
E.P.M was the project leader, collected samples and data, and edited the manuscript. E.P.M and D.B.W. were responsible for project design. D.B.W. collated and analysed the data and wrote the manuscript. E.C.d.P., J.S., R.D.L. and B.R. collected samples and data, made conceptual contributions and edited the manuscript.
Funding information
The project was funded jointly by the National Zoological Gardens of South Africa (NZG) and the Zoological Pathology Programme of the University of Illinois.
Data availability
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
Footnotes
How to cite this article: Woodburn, D.B., Steyl, J., Du Plessis, E.C., Last, R.D., Reininghaus, B. & Mitchell, E.P., 2021, ‘Pathological findings in African buffaloes (Syncerus caffer) in South Africa’, Journal of the South African Veterinary Association 92(0), a2117. https://doi.org/10.4102/jsava.v92i0.2117
References
- Alexander, K.A., Blackburn, J.K., Vandewalle, M.E., Pesapane, R., Baipoledi, E.K. & Elzer, P.H., 2012, ‘Buffalo, bush meat, and the zoonotic threat of brucellosis in Botswana’, PLoS One 7(3), 1–11. 10.1371/journal.pone.0032842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherstone, C., Picozzi, K. & Kalema-Zikusoka, G., 2014, ‘Seroprevalence of Leptospira hardjo in cattle and African buffaloes in south western Uganda’, American Journal of Tropical Medicine and Hygiene 90(2), 288–290. 10.4269/ajtmh.13-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayebazibwe, C., Mwiine, F.N., Tjørnehøj, K., Balinda, S.N., Muwanika, V.B., Ademun Okurut, A.R. et al. , 2010, ‘The role of African buffaloes (Syncerus caffer) in the maintenance of foot-and-mouth disease in Uganda’, BMC Veterinary Research 6, 54. 10.1186/1746-6148-6-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beechler, B.R., Bengis, R., Swanepoel, R., Paweska, J.T., Kemp, A., Van Vuren, P.J. et al. , 2015, ‘Rift Valley fever in Kruger National Park: Do buffalo play a role in the inter-epidemic circulation of virus?’, Transboundary and Emerging Diseases 62(1), 24–32. 10.1111/tbed.12197 [DOI] [PubMed] [Google Scholar]
- Beechler, B.R., Jolles, A.E., Budischak, S.A., Corstjens, P.L.A.M., Ezenwa, V.O., Smith, M. et al. , 2017, ‘Host immunity, nutrition and coinfection alter longitudinal infection patterns of schistosomes in a free ranging African buffalo population’, PLoS Neglected Tropical Diseases 11(12), e0006122. 10.1371/journal.pntd.0006122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengis, R., Govender, D., Lane, E., Myburgh, J., Oberholster, P., Buss, P. et al. , 2016, ‘Eco-epidemiological and pathological features of wildlife mortality events related to cyanobacterial bio-intoxication in the Kruger National Park, South Africa’, Journal of the South African Veterinary Association 87(1), e1–e9. 10.4102/jsava.v87i1.1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengis, R.G., Kriek, N.P.J., Keet, D.F., Raath, P., De Vos, V. & Huchzermeyer, H.F., 1996, ‘An outbreak of bovine tuberculosis in a free-living African buffalo (Syncerus caffer-Sparrman) population in the Kruger National Park: A preliminary report’, Onderstepoort Journal of Veterinary Research 63(1), 15–18. [PubMed] [Google Scholar]
- Bourque, A.C., Fuentealba, I.C., Bildfell, R., Daoust, P.Y. & Hanna, P., 2001, ‘Congenital hepatic fibrosis in calves’, Canadian Veterinary Journal 42(2), 145–146. 10.4141/cjas62-025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breed, D., Meyer, L.C.R., Steyl, J.C.A., Goddard, A., Burroughs, R. & Kohn, T.A., 2019, ‘Conserving wildlife in a changing world: Understanding capture myopathy – A malignant outcome of stress during capture and translocation’, Conservation Physiology 7(1), coz027. 10.1093/conphys/coz027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron, A., Cornelis, D., Foggin, C., Hofmeyr, M. & De Garine-Wichatitsky, M., 2016, ‘African buffalo movement and zoonotic disease risk across transfrontier conservation areas, southern Africa’, Emerging Infectious Diseases 22(2), 277–280. 10.3201/eid2202.140864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisi, M.E., Sibeko, K.P., Collins, N.E., Potgieter, F.T. & Oosthuizen, M.C., 2011, ‘Veterinary parasitology identification of Theileria parva and Theileria sp (buffalo) 18S rRNA gene sequence variants in the African buffalo (Syncerus caffer) in southern Africa’, Veterinary Parasitology 182(2–4), 150–162. 10.1016/j.vetpar.2011.05.041 [DOI] [PubMed] [Google Scholar]
- Clift, S.J., Collins, N.E., Oosthuizen, M.C., Steyl, J.C.A., Lawrence, J.A. & Mitchell, E.P., 2020, ‘The pathology of pathogenic theileriosis in African wild artiodactyls’, Veterinary Pathology 57(1), 24–48. 10.1177/0300985819879443 [DOI] [PubMed] [Google Scholar]
- Coetzer, J.A.W., Thomson, G.R. & Tustin, R.C. (eds.), 2004, Infectious diseases of livestock, 2nd edn., Oxford University Press, Cape Town. [Google Scholar]
- Cossaboom, C.M., Khaiseb, S., Haufiku, B., Katjiuanjo, P., Kannyinga, A., Mbai, K. et al. , 2019, ‘Anthrax epizootic in wildlife, Bwabwata National Park, Namibia’, Emerging Infectious Diseases 25(5), 947–950. 10.3201/eid2505.180867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos, V., Bengis, R.G., Kriek, N.P.J., Michel, A., Keet, D.F., Raath, J.P. et al. , 2001, ‘The epidemiology of tuberculosis in free-ranging African buffalo (Syncerus caffer) in the Kruger National Park, South Africa’, Onderstepoort Journal of Veterinary Research 68(2), 119–130. [PubMed] [Google Scholar]
- De Vos, V. & Turnbull, P.C.B., 2004, ‘Anthrax’, in Coetzer J.A.W., Thomson G.R. & Tustin R.C. (eds.), Infectious diseases of livestock, 2nd edn., pp. 1788–1818, Oxford University Press, Cape Town. [Google Scholar]
- Dräger, N. & Paine, G.D., 1980, ‘Demodicosis in African buffalo (Sycerus caffer) in Botswana’, Journal of Wildlife Diseases 16(4), 521–524. 10.7589/0090-3558-16.4.521 [DOI] [PubMed] [Google Scholar]
- Dubey, J.P., Lane, E.P., Van Wilpe, E., Suleman, E., Reininghaus, B., Verma, S.K. et al. , 2014, ‘Sarcocystis cafferi n. sp. (Protozoa: Apicomplexa) from the African buffalo (Syncerus caffer)’, Journal of Parasitology 100(6), 817–827. 10.1645/13-467.1 [DOI] [PubMed] [Google Scholar]
- Ebedes, H., 1976, ‘Anthrax epizootics in Etosha National Park’, Madoqua 10(2), 99–118. [Google Scholar]
- Eygelaar, D., Jori, F., Mokopasetso, M., Sibeko, K.P., Collins, N.E., Vorster, I. et al. , 2015, ‘Tick-borne haemoparasites in African buffalo (Syncerus caffer) from two wildlife areas in Northern Botswana’, Parasites and Vectors 8, 26. 10.1186/s13071-014-0627-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagbo, S., Coetzer, J.A.W.W. & Venter, E.H., 2014, ‘Seroprevalence of Rift Valley fever and lumpy skin disease in African buffalo (Syncerus caffer) in the Kruger National Park and Hluhluwe-iMfolozi Park, South Africa’, Journal of the South African Veterinary Association 85(1), a1075. 10.4102/jsava.v85i1.1075 [DOI] [PubMed] [Google Scholar]
- Gcebe, N., Rutten, V., Gey van Pittius, N.C. & Michel, A., 2013, ‘Prevalence and distribution of non-tuberculous mycobacteria (NTM) in cattle, African buffaloes (Syncerus caffer) and their environments in South Africa’, Transboundary and Emerging Diseases 60(S1), 74–84. 10.1111/tbed.12133 [DOI] [PubMed] [Google Scholar]
- Gonçalves, L.R., Teixeira, M.M.G., Rodrigues, A.C., Mendes, N.S., Matos, C.A., Pereira, C.L. et al. , 2018, ‘Molecular detection of Bartonella species and haemoplasmas in wild African buffalo (Syncerus caffer) in Mozambique, Africa’, Parasitology Open 4, 1–8. 10.1017/pao.2018.10 [DOI] [Google Scholar]
- Gorsich, E.E., Ezenwa, V.O. & Jolles, A.E., 2014, ‘Nematode – Coccidia parasite co-infections in African buffalo: Epidemiology and associations with host condition and pregnancy’, International Journal of Parasitology 3(2), 124–134. 10.1016/j.ijppaw.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradwell, D.V., Schutte, A.P., Van Niekerk, C.A.W.J. & Roux, D.J., 1977, ‘The isolation of Brucella abortus biotype 1 from African buffalo in the Kruger National Park’, Journal of South African Veterinary Association 48(1), 41–43. [PubMed] [Google Scholar]
- Herr, S. & Marshall, C., 1981, ‘Brucellosis in free-living African buffalo (Syncerus caffer): A serological survey’, Onderstepoort Journal of Veterinary Research 48(3), 133–134. [PubMed] [Google Scholar]
- Hogan, J.N., Miller, W.A., Cranfield, M.R., Ramer, J., Hassell, J., Bosco Noheri, J. et al. , 2014, ‘Giardia in mountain gorillas (Gorilla beringei beringei), forest buffalo (Syncerus caffer), and domestic cattle in Volcanoes National Park, Rwanda’, Journal of Wildlife Diseases 50(1), 21–30. 10.7589/2012-09-229 [DOI] [PubMed] [Google Scholar]
- Jori, F., Alexander, K.A., Mokopasetso, M., Munstermann, S., Moagabo, K. & Paweska, J.T., 2015, ‘Serological evidence of Rift Valley fever virus circulation in domestic cattle and African buffalo in northern Botswana (2010–2011)’, Frontiers of Veterinary Science 2, 63. 10.3389/fvets.2015.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabongo, N. & Van Vuuren, M., 2004, ‘Detection of bovine viral diarrhoea virus in specimens from cattle in South Africa and possible association with clinical disease’, Journal of the South African Veterinary Association 75(2), 90–93. 10.4102/jsava.v75i2.459 [DOI] [PubMed] [Google Scholar]
- Kading, R.C., Borland, E.M., Cranfield, M. & Powers, A.M., 2013, ‘Prevalence of antibodies to alphaviruses and flaviviruses in free-ranging game animals and non-human primates in the greater Congo basin’, Journal of Wildlife Diseases 49(3), 587–599. 10.7589/2012-08-212 [DOI] [PubMed] [Google Scholar]
- Kalema-Zikusoka, G., Bengis, R.G., Michel, A.L. & Woodford, M.H., 2005, ‘A preliminary investigation of tuberculosis and other diseases in African buffalo (Syncerus caffer) in Queen Elizabeth National Park, Uganda’, Onderstepoort Journal of Veterinary Research 72(2), 145–151. 10.4102/ojvr.v72i2.210 [DOI] [PubMed] [Google Scholar]
- Kellerman, T.S., Coetzer, J.A.W., Naude, T.W. & Botha, C.J., 2005, Plant poisonings and mycotoxicoses of livestock of southern Africa, 2nd edn., Oxford University Press, Cape Town. [Google Scholar]
- Laisse, C.J.M., Gavier-Widen, D., Ramis, G., Bila, C.G., Machado, A., Quereda, J.J. et al. , 2011, ‘Characterization of tuberculous lesions in naturally infected African buffalo (Syncerus caffer)’, Journal of Veterinary Diagnostic Investigation 23(5), 1022–1027. 10.1177/1040638711416967 [DOI] [PubMed] [Google Scholar]
- Lane, E.P., 1995, ‘Indicators of health and disease in free-ranging impala’, MPhil thesis, University of Zimbabwe, Harare. [Google Scholar]
- Lawrence, J.A., Foggin, C.M. & Prozesky, L., 2010, ‘Gousiekte in African buffalo (Syncerus caffer)’, Journal of the South African Veterinary Association 81(3), 170–171. 10.4102/jsava.v81i3.142 [DOI] [PubMed] [Google Scholar]
- Mbatha, K.R.., Lane, E.P., Lander, M., Tordiffe, A.S.W. & Corr, S., 2012, ‘Preliminary evaluation of selected minerals in liver samples from springbok (Antidorcas marsupialis) from the National Zoological Gardens of South Africa’, Journal of the South African Veterinary Association 83(1), a119. 10.4102/jsava.v83i1.119 [DOI] [PubMed] [Google Scholar]
- Meltzer, D. & Kock, N., 2012, ‘Stress and capture-related death’, in Kock M.D. & Burroughs R.E. (eds.), Chemical and physical restraint of wild animals, pp. 81–88, International Wildlife Veterinary Services, Greyton, South Africa. [Google Scholar]
- Michel, A.L. & Bengis, R.G., 2012, ‘The African buffalo: A villain for inter-species spread of infectious diseases in southern Africa’, Onderstepoort Journal of Veterinary Research 79(2), a453. 10.4102/ojvr.v79i2.453 [DOI] [PubMed] [Google Scholar]
- Michel, A.L., Bengis, R.G., Keet, D.F., Hofmeyr, M., De Klerk, L.M., Cross, P.C. et al. , 2006, ‘Wildlife tuberculosis in South African conservation areas: Implications and challenges’, Veterinary Microbiology 112(2–4), 91–100. 10.1016/j.vetmic.2005.11.035 [DOI] [PubMed] [Google Scholar]
- Motsi, T.R., Tichiwangana, S.C., Matope, G. & Mukarati, N.L., 2013, ‘A serological survey of brucellosis in wild ungulate species from five game parks in Zimbabwe’, Onderstepoort Journal of Veterinary Research 80(1), a586. 10.4102/ojvr.v80i1.586 [DOI] [PubMed] [Google Scholar]
- Moyo, D.Z., Chakuya, J. & Sungirai, M., 2018, ‘Ixodid ticks of African buffalo (Syncerus caffer), impala (Aepyceros melampus) and elephant (Loxodonta africana) in five protected park estates in the Zambezi valley, Zimbabwe’, Experimental and Applied Acarology 75, 409–417. 10.1007/s10493-018-0275-6 [DOI] [PubMed] [Google Scholar]
- Muma, J.B., Gabriël, S., Munyeme, M., Munang’andu, H.M., Victor, B., Dorny, P. et al. , 2014, ‘Taenia spp. infections in wildlife in the Bangweulu and Kafue flood plains ecosystems of Zambia’, Veterinary Parasitology 205(1–2), 375–378. 10.1016/j.vetpar.2014.07.010 [DOI] [PubMed] [Google Scholar]
- Munang’andu, H.M., Siamudaala, V.M., Matandiko, W., Munyeme, M., Chenbensofu, M., Mwase, E. et al. , 2010, ‘Sarcoptes mite epidemiology and treatment in African buffalo (Syncerus caffer) calves captured for translocation from the Kafue game management area to game ranches’, BMC Veterinary Research 6, 29. 10.1186/1746-6148-6-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musoke, J., Hlokwe, T., Marcotty, T., Plessis, B.J.A. & Michel, A.L., 2015, ‘Spillover of Mycobacterium bovis from wildlife to livestock, South Africa’, Emerging Infectious Diseases 21(3), 448–451. 10.3201/eid2103.131690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndeereh, D., Muchemi, G., Thaiyah, A., Otiende, M., Angelone-Alasaad, S. & Jowers, M.J., 2017, ‘Molecular survey of Coxiella burnetii in wildlife and ticks at wildlife-livestock interfaces in Kenya’, Experimental and Applied Acarology 72, 277–289. 10.1007/s10493-017-0146-6 [DOI] [PubMed] [Google Scholar]
- O’Toole, D., Kelly, E.J., Mcallister, M.M., Layton, A.W., Norrdin, R.W., Russell, W.C. et al. , 2001, ‘Hepatic failure and hemochromatosis of Salers and Salers-cross cattle’, Veterinary Pathology 38(4), 372–389. 10.1354/vp.38-4-372 [DOI] [PubMed] [Google Scholar]
- Pfitzer, S., Last, R., Espie, I. & Van Vuuren, M., 2015, ‘Malignant catarrhal fever: An emerging disease in the African buffalo (Syncerus caffer)’, Transboundary and Emerging Diseases 62(3), 288–294. 10.1111/tbed.12131 [DOI] [PubMed] [Google Scholar]
- Praveena, P.E., Periasamy, S., Kumar, A.A. & Singh, N., 2014, ‘Pathology of experimental infection by Pasteurella multocida serotype A: 1 in buffalo calves’, Veterinary Pathology 51(6), 1109–1112. 10.1177/0300985813516647 [DOI] [PubMed] [Google Scholar]
- Quant, S., Bengis, R.G., Stolte, M., Odening, K. & Bockhardt, I., 1997, ‘Sarcocystis infection of the African buffalo (Syncerus caffer) in the Kruger National Park, South Africa’, Acta Parasitologica 42(2), 68–73. [Google Scholar]
- Ritz, F., Oggin, C.H.F., Akaya, P.I.V.M., Shabalala, P.R.T.T., Miguel, E., Grosbois, V. et al. , 2013, ‘Contacts and foot and mouth disease transmission from wild to domestic bovines in Africa’, Ecosphere 4(4), 1–32. 10.1890/ES12-00239.1 [DOI] [Google Scholar]
- Rodwell, T.C., Kriek, N.P., Bengis, R.G., Whyte, I.J., Viljoen, P.C., De Vos, V. et al. , 2001, ‘Prevalence of bovine tuberculosis in African buffalo at Kruger National Park’, Journal of Wildlife Diseases 37(2), 258–264. 10.7589/0090-3558-37.2.258 [DOI] [PubMed] [Google Scholar]
- Sisson, D., Hufschmid, J., Jolles, A., Beechler, B. & Jabbar, A., 2017, ‘Ticks and tick-borne diseases molecular characterisation of Anaplasma species from African buffalo (Syncerus caffer) in Kruger National Park, South Africa’, Ticks and Tick Borne Diseases 8(3), 400–406. 10.1016/j.ttbdis.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Stepanovic, S., Dimitrijevi, V., Vukovi, D., Daki, I., Savi, B. & Švabic-Vlahovi, M., 2001, ‘Staphylococcus sciuri as a part of skin, nasal and oral flora in healthy dogs’, Veterinary Microbiology 82(2), 177–185. 10.1016/S0378-1135(01)00377-7 [DOI] [PubMed] [Google Scholar]
- Tanner, M., Inlameia, O., Michel, A., Maxlhuza, G., Pondja, A., Fafetine, J. et al. , 2014, ‘Bovine tuberculosis and brucellosis in cattle and African buffalo in the Limpopo National Park, Mozambique’, Transboundary and Emerging Diseases 62(6), 632–638. 10.1111/tbed.12210 [DOI] [PubMed] [Google Scholar]
- Tavalire, H.F., Beechler, B.R., Buss, P.E., Gorsich, E.E., Hoal, E.G., Johannie, R. et al. , 2018, ‘Context-dependent costs and benefits of tuberculosis resistance traits in a wild mammalian host’, Ecology and Evolution 8(24), 12712–12726. 10.1002/ece3.4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, W.A., Skinner, J.D. & Boomker, J., 2013, ‘Nematodes of the small intestine of African buffaloes, Syncerus caffer, in the Kruger National Park, South Africa’, Onderstepoort Journal of Veterinary Research 80(1), a562. 10.4102/ojvr.v80i1.562 [DOI] [PubMed] [Google Scholar]
- Thomson, G.R. & Bastos, A.D., 2004, ‘Foot and mouth disease’, in Coetzer J.A.W. & Tustin R.C. (eds.), Infectious diseases of livestock, pp. 1324–1365, Oxford University Press, Cape Town. [Google Scholar]
- Vosloo, W., Thompson, P.N.N., Botha, B., Bengis, R.G.G. & Thomson, G.R.R., 2009, ‘Longitudinal study to investigate the role of impala (Aepyceros melampus) in foot-and-mouth disease maintenance in the Kruger National Park, South Africa’, Transboundary and Emerging Diseases 56(1–2), 18–30. 10.1111/j.1865-1682.2008.01059 [DOI] [PubMed] [Google Scholar]
- Wekesa, S.N., Sangula, A.K., Belsham, G.J., Tjornehoj, K., Muwanika, V.B., Gakuya, F. et al. , 2015, ‘Characterisation of recent foot-and-mouth disease viruses from African buffalo (Syncerus caffer) and cattle in Kenya is consistent with independent virus populations’, BMC Veterinary Research 11, 17. 10.1186/s12917-015-0333-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, J., 2014, ‘Pathology of fatal lineage 1 and 2 West Nile virus infections in horses in South Africa’, Journal of the South African Veterinary Association 85(1), a1105. 10.4102/jsava.v85i1.1105 [DOI] [PubMed] [Google Scholar]
- Williams, J.H., Van Dyk, E., Nel, P.J., Lane, E., Van Wilpe, E., Bengis, R.G. et al. , 2011, ‘Pathology and immunohistochemistry of papillomavirus-associated cutaneous lesions in Cape mountain zebra, giraffe, sable antelope and African buffalo in South Africa’, Journal of the South African Veterinary Association 82(3), 97–106. 10.4102/jsava.v82i3.59 [DOI] [PubMed] [Google Scholar]
- Wolf-Jäckel, G.A., Hansen, M.S., Larsen, G., Holm, E., Agerholm, J.S. & Jensen, T.K., 2020, ‘Diagnostic studies of abortion in Danish cattle 2015–2017’, Acta Veterinaria Scandinavia 62, 1. 10.1186/s13028-019-0499-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolhuter, J., Bengis, R.G., Reilly, B.K. & Cross, P.C., 2009, ‘Clinical Demodicosis in African Buffalo (Syncerus caffer) in the Kruger National Park’, Journal of Wildlife Diseases 45(2), 502–504. 10.7589/0090-3558-45.2.502 [DOI] [PubMed] [Google Scholar]
- Yoshikawa, H., Fukuda, T., Oyamada, T. & Yoshikawa, T., 2002, ‘Congenital hepatic fibrosis in a newborn calf’, Veterinary Pathology 39(1), 143–145. 10.1354/vp.39-1-143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.