Abstract
Aging of the arteries is characterized by increased large artery stiffness and impaired endothelium dependent dilation. T cells contribute to hypertension in acute rodent models but whether they contribute to chronic age-related arterial dysfunction is unknown. To determine whether T cells directly mediate age-related arterial dysfunction, we examined large elastic artery and resistance artery function in young (4-6 months) and old (22-24 months) wild type mice treated with anti-CD3 F(ab’2) fragments to deplete T cells (150μg, i.p. every 7 days for 28 days) or isotype control fragments. Old mice exhibited greater numbers of T cells in both aorta and mesenteric vasculature when compared to young mice. Old mice treated with anti-CD3 fragments exhibited depletion of T cells in blood, spleen, aorta and mesenteric vasculature. Old mice also exhibited greater numbers of aortic and mesenteric IFN-γ and TNF-α producing T cells when compared to young. Old control mice exhibited greater large artery stiffness and impaired resistance artery endothelium dependent dilation in comparison to young mice. In old mice, large artery stiffness was ameliorated with anti-CD3 treatment. Anti-CD3 treated old mice also exhibited greater endothelium dependent dilation compared to age-matched controls. We also examined arterial function in young and old Rag-1−/− mice, which lack lymphocytes. Rag-1−/− mice exhibited blunted increases in large artery stiffness with age compared to wild type mice. Old Rag-1−/− mice also exhibited greater endothelium dependent dilation compared to old wild type mice. Collectively, these results demonstrate that T cells play an important role in age-related arterial dysfunction.
Keywords: endothelium, vascular, immune system, lymphocytes, aorta, mesentery
INTRODUCTION:
Aging is the most predictive risk factor for cardiovascular disease (Lakatta & Levy, 2003) and the majority of cardiovascular diseases are diseases of the arteries. Two major hallmarks of arterial aging are: 1) increased large artery stiffness, and 2) impaired endothelium dependent dilation, primarily mediated by a loss of endothelial-derived nitric oxide (NO) (Gerhard et al., 1996; Lakatta & Levy, 2003; Blackwell et al., 2004; Donato et al., 2007). Both age-related increases in large artery stiffness and impairments in endothelium dependent dilation are, in part, mediated by increased arterial superoxide (Blackwell et al., 2004; Donato et al., 2007; Trott et al., 2011; Gioscia-Ryan et al., 2018). Specifically, superoxide reacts with NO, generating peroxynitrite and decreasing NO bioavailability which can cause both impairments in endothelium dependent dilation and increased large artery stiffness (Fitch et al., 2001; Blackwell et al., 2004; Fleenor et al., 2012). Inflammation has been implicated in age-related arterial reactive oxygen species production and subsequent dysfunction; however, the vast majority of investigations have focused on inflammation of the vascular wall itself (Wang et al., 2007; Donato et al., 2008; Pierce et al., 2009; Morgan et al., 2013). In contrast, whether cells of the immune system induce arterial inflammation with advancing age is less well understood.
T cells have been shown to contribute to endothelial dysfunction and large artery stiffening in acute rodent models of hypertension (Guzik et al., 2007; De Miguel et al., 2010; Wu et al., 2016; Pan et al., 2020), as well as in the development of atherosclerosis (Elhage et al., 2004). In contrast to these acutely induced models of rodent hypertension and atherosclerosis, aging is more representative of human physiology where arterial function declines over years rather than days or weeks. Further, large artery stiffening and endothelial dysfunction precede the development of frank cardiovascular diseases like hypertension and atherosclerosis (Liao et al., 1999; Ras et al., 2013). In this investigation, we sought to determine whether T cells contribute to arterial aging independent of overt cardiovascular disease.
There is evidence that T cell specific aging contributes to cardiovascular risk, as T cells with an aged phenotype are more prevalent in middle-aged humans with hypertension (Youn et al., 2013). Arteries from older adults also exhibit greater CCL2 (an important T cell recruiting chemokine) production (Wang et al., 2007; Morgan et al., 2013). Intriguingly, patients with rheumatoid arthritis, an autoimmune disease that features premature T cell aging, are at greater risk for cardiovascular disease even when controlling for traditional risk factors (del Rincon et al., 2001).
We have found that T cells accumulate in aorta and the mesenteric vascular arcade in old mice (Lesniewski et al., 2011; Trott et al., 2018). Despite these observational studies, it is unknown whether T cells play a direct role in age-related arterial dysfunction prior to the onset of frank arterial diseases. Therefore, this investigation was designed to test the hypothesis that T cells directly contribute to age-related arterial dysfunction.
METHODS AND MATERIALS:
Ethical Approval and Animals:
All animal experiments conformed to the Guide and Use of Laboratory Animals and were approved by the University of Utah, Veteran’s Affairs Medical Center-Salt Lake City (VAMC-SLC) or the University of Texas at Arlington Animal Care and Use Committees. All experiments comply with the ethics policies of J Physiol.
Young male (4-6 month) C57BL/6 mice were obtained from Charles River Inc. and old (22-24 months) male C57BL/6 mice were obtained from the National Institute of Aging (NIA) colony maintained by Charles River Inc. For studies of genetic lymphocyte deficiency B6.129S7-Rag1tm1Mom/J (Rag-1−/−), male and female mice were obtained from Jackson Laboratories and were bred and aged at the VAMC-SLC animal facility. Male and female C57BL/6 mice served as controls. All mice were housed under specific pathogen free conditions in standard mouse cages on a 12:12 light:dark cycle with ad libitum access to food and water in the animal facility at either the VAMC-SLC or at University of Texas at Arlington. Mice were euthanized by a terminal cardiac puncture followed by secondary bilateral thoracotomy while under isoflurane anesthesia. Unless otherwise stated, chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
T cell depletion:
To deplete T cells, young (n = 24) and old (n = 29) mice were treated with anti-CD3 F(ab’)2 fragments (BioXCell catalog #BE0001-1FAB, 150μg i.p., once every 7 days, for 28 days). This regimen has been shown to deplete T cells both in the spleen (a major T cell reservoir) (Hirsch et al., 1990) and in peripheral tissues (Winer et al., 2009). Control young (n = 24) and old (n = 28) mice were treated with a matched regimen of isotype control F(ab’)2 fragments (BioXCell catalog #BE0091-FAB). On day 28 the mice were euthanized. Mice that died prior to day 28 were excluded from final analyses.
Blood Pressure:
Before commencing antibody fragment treatment and 2 to 3 days prior to euthanasia, conscious blood pressure was assessed non-invasively in a subset of mice using the tail volume pressure recording method (CODA, Kent Scientific) as described previously (Donato et al., 2013).
Pulse Wave Velocity:
Before antibody fragment treatment and one to two days prior to euthanasia, large artery stiffness was assessed by measuring aortic PWV as described previously (Machin et al., 2020). Briefly, mice were anesthetized under 2% isoflurane and maintained via nose-cone. Mice were secured in a supine position on a heating board (~35° C) to maintain body temperature. Velocities were measured with 4 mm piezoelectric crystal, 20-MHz Doppler probes (Indus Instruments, Webster, TX, USA) at the transverse aortic arch and ~3 cm distal at the abdominal aorta and collected using WinDAQ Pro+ software (DataQ Instruments, Akron, OH, USA). Absolute pulse arrival times were indicated by the sharp upstroke, or foot, of each velocity waveform analyzed with WinDAQ Waveform Browser (DataQ Instruments). Aortic pulse-wave velocity was then calculated as the quotient of the separation distance, assessed to the nearest half millimeter by engineering caliper (typically ~3 cm), and the difference in absolute arrival times.
Flow cytometry:
Following euthanasia, to remove circulating leukocytes from arteries, the chest cavity was opened and the right atrium was nicked. A cannula was placed in the left ventricle and the animals were perfused with saline + 10 U/ml of heparin at physiological pressure until the effluent was cleared of blood. Aortas were dissected from the aortic arch to the diaphragm and lymph nodes were removed. Blood vessels and perivascular tissue of the mesenteric vascular arcade was dissected away from the intestinal wall with care to avoid puncturing the wall and to exclude mesenteric lymph nodes. Perivascular adipose tissue around both the aorta and mesenteric vascular arcade was included as this is where arterial immune cells accumulate (Guzik et al., 2007; Lesniewski et al., 2011).
Following dissection, spleens, aortas, and mesenteric vascular arcades were digested using collagenase type I (Worthington, 450 U/ml), and DNAse (Worthington, 0.1 mg/ml) dissolved in DPBS buffer containing calcium and magnesium for 30 min at 37° C. The tissues were further dispersed using repeated pipetting and the resultant homogenate was passed through a 70 μm sterile filter, yielding single-cell suspensions. Dead cells were labeled with Tonbo Ghost Dye and excluded from analysis. Single cell suspensions were labeled with the following anti-mouse antibodies at a 1:100 concentration: violetfluor450-CD45, Tonbo #75-0454 (total leukocytes), APC-CD3, Tonbo #20-0032 (pan T cells), FITC-CD4, Tonbo #30-0041 (T helper cells), PE-Cy7-CD8, Tonbo #60-0081 (cytotoxic T cells), PerCP Cy5.5-CD44, Tonbo #65-00441 (naïve vs memory), and APC-Cy7-CD62L Tonbo #25-0621 (central vs effector).
To assess T cell cytokine production single cell suspensions were stimulated with phorbol 12-myristate 13-acetate (PMA, 10 ng/ml) and ionomycin (1 μg/ml) for 6 hours. During this incubation, protein transport out of the golgi apparatus was blocked with brefeldin A (10 μg/ml). Following stimulation cells were labeled with the following anti-mouse antibodies: PE/Dazzle-CD45, Biolegend #109846, APC-CD3, Tonbo #20-0032, FITC-CD4, Tonbo #30-0041 and PE-Cy5-CD8 Tonbo #55-0081. Following cell surface staining cells were fixed in 2% paraformaldehyde and permeablized with Intracellular Staining Permeabilization Wash Buffer (Biolegend). Cells were then labeled with PE-Cy7-IFN-γ, Tonbo #60-7311 and PerCPCy5.5-TNF-α, Biolegend #506321.
In separate experiments B cells and macrophages were assessed with violetfluor450-CD45 (total leukocytes), APC Cy7-CD19, Biolegend #115529 (B cells), PE-CD64, Biolegend #139303 (macrophages), FITC-CD11c, Tonbo #35-0114 (exclusion of dendritic cells), APC-CD206, Biolegend #141707 (M1/M2 macrophage phenotype) (Zamarron et al., 2017). Cell subpopulations were assessed on a 3 laser BD FACS Canto II or a 3 laser BD FACS Melody. The “fluorescence minus one” technique was used to establish gating, as described previously (Trott et al., 2014).
Gene expression:
Gene expression for superoxide producing enzymes and superoxide dismutases was assessed by quantitative PCR. Following euthanasia, aortas from young and old, isotype and anti-CD3 treated mice were dissected and perivascular adipose tissue was removed. RNA was extracted using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. mRNA was converted into cDNA by using QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer’s protocol. Quantitative PCR was performed on 96-well plates by using RT2 SYBR Green qPCR Master mix (Qiagen) with the Bio-Rad CFX™ Real-Time System. Expression of the genes was normalized to 18s and are presented as fold change compared to young isotype as calculated by the ΔΔCt method (Livak & Schmittgen, 2001). The primers employed for qPCR are shown in Table 1.
Table 1.
Primers used for qPCR
| Primer | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| Xo | GAATGGCAAAAAGGTGGTGGA | AGCAACATGATGCAAGGAGC |
| Nox2 | CGCATGCCTTTGAGTGGTTT | ACGCCTATTGTGGTGTTAGGG |
| Sod1 | AACCAGTTGTGTTGTCAGGAC | CCACCATGTTTCTTAGAGTGAGG |
| Sod2 | CAGACCTGCCTTACGACTATGG | CTCGGTGGCGTTGAGATTGTT |
| Sod3 | CCTTCTTGTTCTACGGCTTGC | TCGCCTATCTTCTCAACCAGG |
| 18s | TAGAGGGACAAGTGGCGTTC | CGCTGAGCCAGTCAGTGT |
Mesenteric Artery Endothelium Dependent Dilation:
To assess endothelium dependent dilation, 2nd order mesenteric arteries were gently cleared of adipose and connective tissue and cannulated in the stage of a pressure myograph (DMT Inc, Atlanta, GA, USA) in physiological saline solution. Arteries were pressurized to an intraluminal pressure of 68 cmH2O and allowed to equilibrate for 60 min. As mesenteric arteries from C57BL/6 mice exhibit a blunted myogenic response with age (Gros et al., 2002), arteries were preconstricted using phenylephrine (≤ 2 μM). Arteries with leaks or ones that did not constrict at least 20% in response to phenylephrine were discarded. Dilation was assessed in response to increasing doses of acetylcholine (ACh,10−9-10−4 M), two minutes per dose. To determine the contribution of NO, the ACh dose response was performed in the presence of L-NAME (0.1 mM) (Durrant et al., 2009; Lesniewski et al., 2009). Superoxide-mediated suppression of endothelium dependent dilation was assessed by repeating the ACh concentration response curves in the presence of the superoxide dismutase mimetic, TEMPOL (1 mM). Endothelium independent dilation was assessed by dose response to sodium nitroprusside (SNP, 10−9-10−3 M), two minutes per dose (Muller-Delp et al., 2002; Durrant et al., 2009). Vessel diameters were measured by MyoView software (DMT, Inc., Atlanta, GA, USA) and the maximal diameter during the last minute of each dose was recorded, consistent with previous methods (Muller-Delp et al., 2002; Durrant et al., 2009; Lesniewski et al., 2009; Trott et al., 2009; Donato et al., 2012). Maximal artery passive diameter was assessed after incubation in Ca2+ free physiological saline for 30 minutes. All dose–response data are presented as percent of possible dilation, calculated as [(Ddose – DB)/(DP - DB)] x 100, where Ddose is the measured diameter for a given dose, DB is baseline tone/preconstricted diameter before an intervention was started, and DP is maximal passive diameter. Baseline tone was calculated as (DB/DP) x 100.
Statistical Analyses:
Personnel were blinded to the treatment group until after data collection was complete. To assess group differences in immune cell and gene expression outcomes, a two-way ANOVA was employed using age and antibody fragment treatment as fixed factors. In cases where a significant age x treatment interaction occurred, we employed Tukey’s post hoc tests to determine group differences. For experiments that compare young and old mice, an independent samples T test was employed. To assess group differences in PWV, heart rate, blood pressure, endothelium dependent dilation and endothelium independent dilation, a repeated measures ANOVA was employed using time or dose and group as factors. In cases where a significant time or dose x group interaction occurred, we employed either Sidak’s (2 groups) or Tukey’s (more than 2 groups) post hoc tests to determine group differences and/or main effects of dose. To compare PWV across the lifespan, slopes were compared using linear regression. Sample size (n) is the number of individual animals per group unless otherwise noted in the figure legend. Statistics were calculated using Graphpad Prism versions 8 and 9. Data are reported as mean ± standard deviation. P values ≤ 0.05 were considered statistically significant.
RESULTS:
Efficacy of T cell depletion
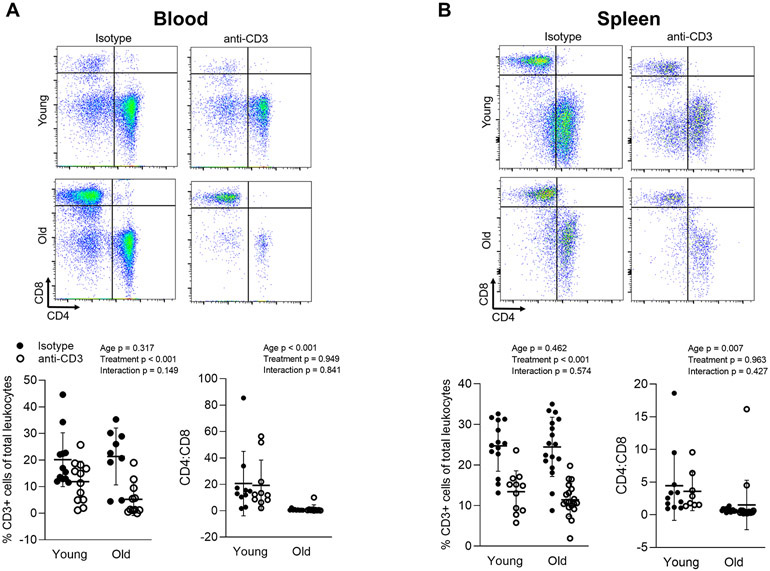
We employed anti-CD3 F(ab’)2 fragments to deplete T cells in young (4-6 months) and old (22-24 months) C57BL/6 mice. Out of 105 total mice randomized to isotype or anti-CD3 treatment, 3 young isotype, 2 young anti-CD3, 5 old isotype and 4 old anti-CD3 died during the 28 day treatment period. We examined total T cells (CD3) as well as CD4 and CD8 T cells in the blood and spleen using flow cytometry after 28 days of treatment with anti-CD3 F(ab’)2 fragments or isotype control fragments. In concert with previous reports (Hirsch et al., 1990), we found lower proportions of CD3 T cells in blood and spleen of both young and old anti-CD3 treated animals (Figure 1A & B). We also observed inverted CD4-to-CD8 ratios in old animals; this inverted ratio was not altered by T cell depletion (Figure 1A & B).
Figure 1: anti-CD3 F(ab’)2 Fragment treatment results in depletion of T cells in the blood and spleen.
Blood (100 μL) from young isotype (n = 10), young anti-CD3 (n = 10), old isotype (n = 9), old anti-CD3 mice (n = 10) mice was directly stained. Spleens from young isotype (n = 11-14), young anti-CD3 (n = 8-11), old isotype (n = 15-17), old anti-CD3 mice (n = 18-19) were enzymatically digested and passed through a cell strainer and then stained for CD45 (total leukocytes), CD3 (pan T cells), CD4 and CD8. Percentages of CD3+ cells and CD4-to-CD8 ratio in (A) blood and (B) spleen were assessed by flow cytometry. A two-way ANOVA was employed to assess the effects of age and anti-CD3 treatment, p values for age, treatment and the age x treatment interaction are inset on each panel. Data are shown as mean ± standard deviation, n represents the number of independent animals in each group.
Effects of T cell depletion on large elastic arteries
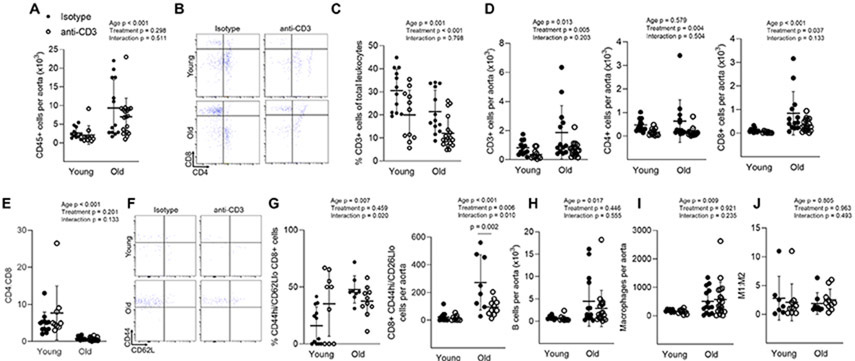
We next assessed aortic immune cell accumulation. Aortas from old mice exhibited greater total immune cell (CD45) accumulation when compared to young (Figure 2A). Anti-CD3 treatment did not alter aortic total immune cell accumulation in either young or old mice. T cells comprised a smaller percentage of total immune cells with both age and anti-CD3 treatment (Figure 2C). Old mice exhibited greater total aortic T cell (CD3) accumulation (Figure 2B & D). The increase in total aortic CD3 cell numbers was primarily driven by an increase in CD8 T cells (Figure 2B & D). Similar to blood and spleen ratios, the CD4:CD8 ratio was lower in aortas from old mice and not affected by anti-CD3 treatment (Figure 2E). Anti-CD3 treatment resulted in fewer aortic total, CD4 and CD8 T cells in both young and old mice when compared to controls (Figure 2B-D). In old mice, aortic CD8 effector memory (CD44hi/CD62Llo) cells made up a greater proportion of CD8 cells and were greater in number compared to young mice (Figure 2F & G). Anti-CD3 treatment resulted in blunted CD8 effector memory cell numbers (Figure 2F & G).
Figure 2: Aortic T cell accumulation with age and anti-CD3 F(ab’)2 Fragment treatment.
Single cell suspensions of thoracic aortas from young isotype (n = 7-12), young anti-CD3 (n = 9-11), old isotype (n = 8-14), old anti-CD3 mice (n = 10-19) were stained with antibodies against CD45 (total leukocytes), CD3 (pan T cells), CD4, CD8, CD44 (memory) and CD62L (central vs. effector). (A) Number of total leukocytes per aorta. (B) Sample aortic CD4 and CD8 flow cytometry plot. (C) Aortic pan, CD4 and CD8 T cell counts. (D) Percentage of CD3 cells out of total aortic immune cells. (E) Aortic CD4:CD8 ratio. (F) Sample aortic naïve (CD44lo) vs memory (CD44hi) flow cytometry plots. (G) Aortic CD8 effector memory T cell proportion and counts. To assess aortic macrophage and B cell accumulation, thoracic aorta single cell suspensions were stained for CD45 (total leukocytes), CD19 (B cells), CD64 (macrophages), CD11c (exclusion of dendritic cells) and CD206 (M1/M2 macrophage phenotype. (H) Aortic B cell counts. (I) Aortic macrophage counts. (J) macrophage M1:M2 ratio. A two-way ANOVA was employed to assess the effects of age and anti-CD3 treatment, p values for age, treatment and the age x treatment interaction are inset on each panel. When a significant age x treatment interaction occurred Tukey’s post hoc test was employed to determine group differences. Significant post hoc test p values are included on the panel with a horizontal line indicating the group comparison. Data are shown as mean ± standard deviation, n represents the number of independent animals in each group.
To determine whether T cell depletion might alter aortic recruitment and/or retention of other immune cells types, we also assessed aortic B cells and macrophages. Aortas from old mice exhibited greater B cell and macrophage numbers than young controls (Figure 2H & I); anti-CD3 treatment did not significantly alter aortic macrophage or B cell accumulation. There was also no alteration in M1/M2 macrophage phenotype with either age or anti-CD3 treatment (Figure 2J).
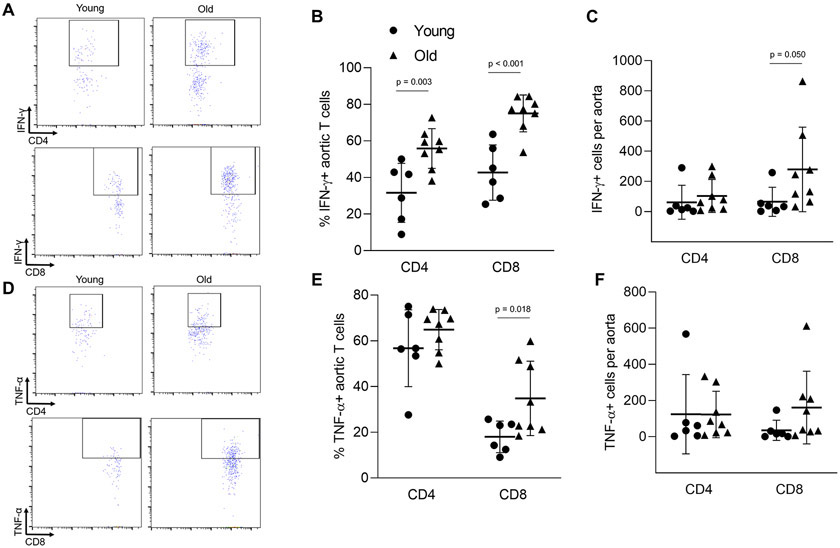
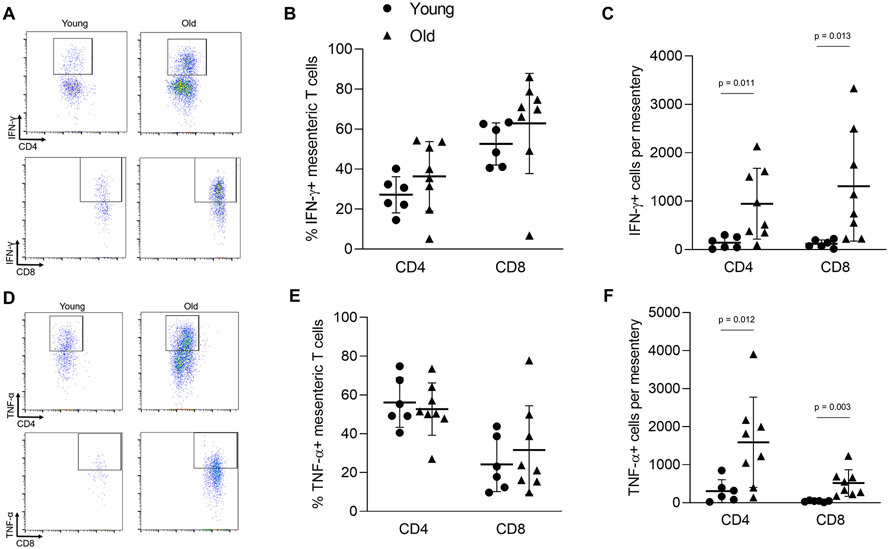
We next sought to determine whether the inflammatory phenotype of arterial T cells is altered with age. We found that aging resulted in a greater proportion of aortic CD4 and CD8 T cells that produced interferon (IFN)-γ and greater total number of CD8 IFN-γ producing cells (Figure 3A-C). We also observed a greater proportion of CD8 T cells producing tumor necrosis factor (TNF)-α in aortas of old mice (Figure 3D-F). The proportions and numbers of aortic CD4 T cells producing TNF-α were not altered (Figure 3D-F).
Figure 3: Aging results in an enhanced proinflammatory phenotype of aortic accumulating T cells.
Single cell suspensions of thoracic aortas from young (n = 6) and old (n = 8) mice were activated in vitro and stained for CD45 (total leukocytes), CD3 (pan T cells), CD4, CD8, interferon (IFN)-γ and tumor necrosis factor (TNF)-α. (A) Sample IFN-γ flow cytometry plots. (B) Proportion and (C) number of IFN-γ producing T cells. (D) Sample TNF-α flow cytometry plots. (E) Proportion and (F) number of TNF-α producing T cells. Group differences were assessed with an independent samples T test, p values are included on the panel with a horizontal line indicating the group comparison. Data are shown as mean ± standard deviation, n represents the number of independent animals in each group.
To determine whether T cells play a role in large artery stiffening, we assessed aortic pulse wave velocity (PWV) before and after anti-CD3 treatment. Before anti-CD3 treatment, old mice exhibited elevated PWV when compared to young mice (Figure 4A). There was an effect of time but not treatment on PWV in young mice (Figure 4B). In old mice, anti-CD3 treatment significantly decreased PWV when compared to old controls (Figure 4C). Heart rates during measurement of PWV were similar between old anti-CD3 treated mice (475 ± 55 beats per min (BPM) pre vs. 451 ± 55 BPM post, n = 12) and old controls (465 ± 40 BPM pre vs. 456 ± 96 BPM post, n = 13) as assessed by repeated measures ANOVA, (time effect p = 0.219, treatment effect p = 0.892, interaction p = 0.580; data are mean ± SD). Neither old mice treated with anti-CD3 fragments (117 ± 20 mmHg pre vs 125 ± 35 mmHg post, n = 14) nor old controls (132 ± 19 mmHg pre, vs. 140 ± 21 mmHg post n = 14), exhibited altered systolic tail cuff blood pressure as assessed by repeated measures ANOVA (time effect p = 0.748, treatment effect p = 0.143, interaction p = 0.900; data are mean ± SD).
Figure 4: T cell depletion reverses age-related increases in large artery stiffness.
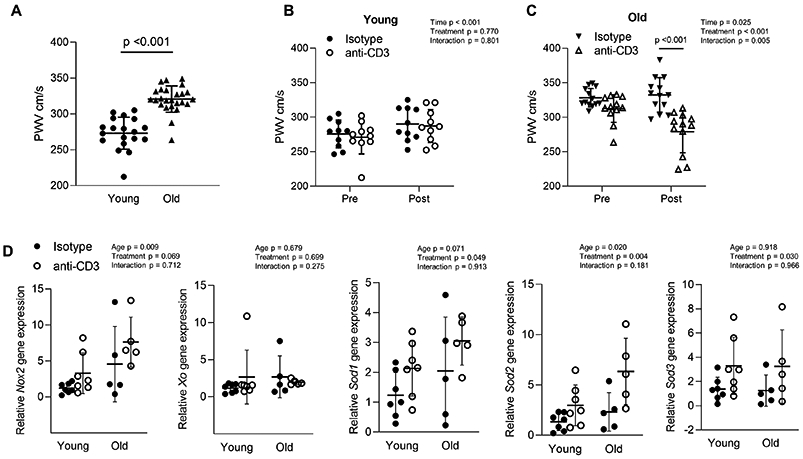
Aortic pulse wave velocity (PWV) was assessed in young (n = 19) and old (n = 25) (A) before anti-CD3 treatment and an independent samples T test was used to assess group differences. p values are included on the panel with a horizontal line indicating the group comparison. PWV was assessed before and after anti-CD3 F(ab’)2 fragment treatment in (B) young (n = 10 per group) and (C) old mice (n = 12-13 per group). A repeated measures ANOVA was used to assess the effect of time, treatment and time x treatment interaction, with p values inset on each panel. When a significant age x treatment interaction occurred Sidak’s post hoc test was employed to determine group differences. Significant post hoc test p values are included on the panel with a vertical line indicating the group comparison. (D) Gene expression of Nox2, Xanthine Oxidase (Xo), Superoxide Dismutase isoforms 1-3 (Sod1, 2 & 3) from aortas of young isotype (n = 7), young anti-CD3 (n = 7) old isotype (n = 5) and old anti-CD3 treated (n = 5) mice. Gene expression data are expressed as fold change compared to young isotype calculated using the ΔΔCt method. Two-way ANOVA was employed to assess the effects of age and anti-CD3 treatment. p values for age, treatment and the age x treatment interaction are inset on each panel. Data are shown as mean ± standard deviation, n represents the number of independent animals in each group.
As superoxide anion plays a role in large artery stiffening, we examined aortic gene expression of enzymes responsible for superoxide production (Xanthine Oxidase, Xo and NADPH Oxidase, Nox2) and superoxide scavenging (Superoxide Dismutase, Sod1, Sod2 and Sod3). We found that Xo gene expression was not altered with age or anti-CD3 treatment (Figure 4D). Old mice exhibited greater Nox2 gene expression compared to young regardless of anti-CD3 treatment (Figure 4D). Anti-CD3 treatment resulted in greater gene expression of Sod1, Sod2 and Sod3 regardless of age group (Figure 4D).
Effects of T cell depletion on the mesenteric arcade
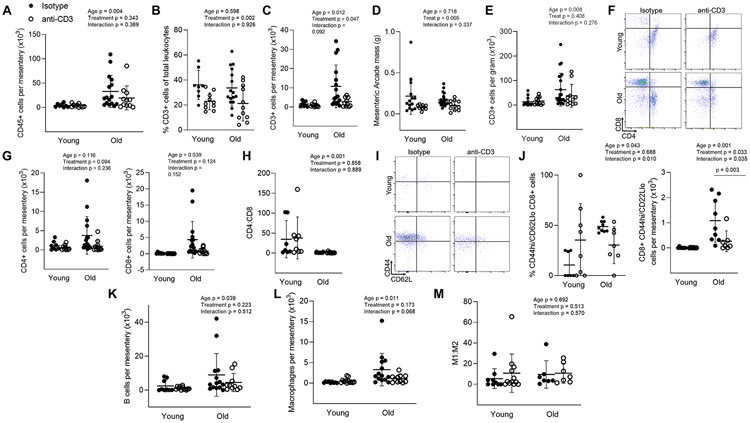
We next assessed immune cell accumulation in the mesenteric vascular arcade. Similar to aorta, total immune cell (CD45) accumulation was greater in the mesenteric arcade of old mice compared to young (Figure 5A). There was no effect of anti-CD3 treatment on total immune cell accumulation (Figure 5A). The proportion of CD3 cells in the mesenteric arcade was not altered with age but was blunted with anti-CD3 treatment (Figure 5B). The mesenteric vascular arcade of old mice exhibited greater pan T (CD3) cell counts compared to young mice (Figures 5C). Anti-CD3 treatment resulted in lower CD3 cell counts in the mesenteric arcade of older mice (Figure 5B & C). To determine whether age-related T cell accumulation is related to mesenteric adipose mass, we assessed mesenteric arcade mass (primarily adipose) and found that it was not altered with age but was blunted with anti-CD3 treatment (Figure 5D). When normalized to mesenteric mass, the age-related accumulation of T cell is preserved. However, anti-CD3 treatment did not result in lower mesenteric T cell numbers when normalized to tissue mass (Figure 5E). We next assessed the subtype of T cells in the mesentery and found that aging resulted in greater accumulation of CD8 T cells in the mesentery and, that despite depleting pan T cells (Figure 5C), anti-CD3 treatment did not result in significant blunting of CD4 (p = 0.094) or CD8 (p = 0.124) cells separately (Figures 5 F & G). Similar to blood, spleen and aorta, CD4:CD8 ratios were blunted in the mesenteric arcade of older animals, and these ratios were not altered by anti-CD3 treatment (Figure 5H). We next assessed CD8 effector memory (CD44hi/CD62Llo) cells in the mesenteric arcade. These cells were almost completely absent in young animals (Figure 5I &J). Old mice exhibited both a greater proportion and number of mesenteric CD8 effector memory cells, and the age-related accumulation of CD8 effector memory cells was ameliorated by anti-CD3 treatment (Figure 5I &J).
Figure 5: Mesenteric T cell accumulation with age and anti-CD3 F(ab’)2 Fragment treatment.
Single cell suspensions of the mesenteric vascular arcade from young isotype (n = 8-14), young anti-CD3 (n = 10-12), old isotype (n = 9-21) and old anti-CD3 mice (n = 7-11) (excluding lymph nodes) were stained for CD45 (total leukocytes), CD3 (pan T cells), CD4, CD8, CD44 (memory) and CD62L (central vs. effector). (A) Number of total leukocytes per mesentery. (B) Proportion of CD3 cells out of all mesenteric leukocytes. (C) Mesenteric CD3 cell counts. (D) Mesenteric arcade mass. (E) Mesenteric CD3 cell counts normalized to tissue mass. (F) Sample CD4 and CD8 flow cytometry plot. (G) Mesenteric CD4 (left) and CD8 (right) T cell counts. (H) Mesenteric CD4:CD8 ratio. (I) Sample mesenteric naïve (CD44lo) vs memory (CD44hi) flow cytometry plots. (J) Proportion of mesenteric (left) and counts (right) of CD8 CD44hi/CD62Llo effector memory T cell counts. To assess mesenteric macrophage and B cell accumulation, mesenteric single cell suspensions were stained for CD45 (total leukocytes), CD19 (B cells), CD64 (macrophages), CD11c (exclusion of dendritic cells) and CD206 (M1/M2 macrophage phenotype. (K) mesenteric B cell counts. (L) mesenteric macrophage counts. (M) macrophage M1:M2 ratio. Two-way ANOVA was employed to assess the effects of age and anti-CD3 treatment. p values for age, treatment and the age x treatment interaction are inset on each panel. When a significant age x treatment interaction occurred Tukey’s post hoc test was employed to determine group differences. Significant post hoc test p values are included on the panel with a horizontal line indicating the group comparison. Data are shown as mean ± standard deviation, n represents the number of independent animals in each group.
We also assessed macrophage and B cell accumulation in the mesenteric vascular arcade. There was an effect of age, but no treatment effect on mesenteric B cell accumulation (Figure 5K). Similarly, mesenteric arcades from old mice had greater macrophage numbers compared to those from young, but there was no effect of anti-CD3 treatment on macrophage accumulation (Figure 5L). Macrophage M1/M2 phenotype was not altered with age or anti-CD3 treatment (Figure 5M).
We assessed T cell cytokine production in cells from the mesentery and found that aging did not alter the proportions of T cells producing either IFN-γ or TNF-α (Figure 6A & B, D & E). In contrast, mesenteries from old mice exhibited greater absolute numbers of CD4 and CD8, IFN-γ and TNF-α producing cells (Figure 6A & C, D & F).
Figure 6: Aging results in an enhanced proinflammatory phenotype of mesenteric accumulating T cells.
To assess cytokine production, mesenteric single cell suspensions from young (n = 6) and old (n = 8) mice were activated in vitro. Cells were then stained for CD45 (total leukocytes), CD3 (pan T cells), CD4, CD8, interferon (IFN)-γ and tumor necrosis factor (TNF)-α. (A) Sample IFN-γ flow cytometry plots. (B) Proportion and (C) number of IFN-γ producing T cells. (D) Sample TNF-α flow cytometry plots. (E) Proportion and (F) number of TNF-α producing T cells. Group differences were assessed with an independent samples T test. p values are included on the panel with a horizontal line indicating the group comparison. Data are shown as mean ± standard deviation, n represents the number of independent animals in each group.
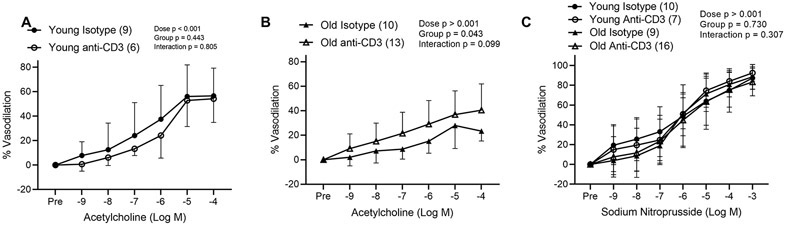
Following antibody fragment treatment, we assessed endothelium dependent dilation in 2nd order mesenteric arteries. Arteriolar diameter did not differ with age or anti-CD3 treatment, nor did initial constrictor tone prior to ACh dose responses (Table 2). We found that old isotype control mice exhibited significantly blunted dilation (maximal dilation of 32.8 ± 14.4) in response to ACh compared to young isotype control mice (maximal dilation of 62.7 ± 20.2, p = 0.001, as compared by independent samples T test, data are means ± SD) (Figure 7A & B). Anti-CD3 treatment did not alter endothelium dependent dilation in young mice (Figure 7A), but old anti-CD3 treated mice exhibited greater endothelium dependent dilation compared to old controls (Figure 7B). Arteries from old mice exhibited blunted preconstriction prior to SNP dose responses (Table 2) but neither age nor treatment effected endothelium independent dilation (Figure 7C).
Table 2.
Characteristics of mesenteric arteries from isotype and anti-CD3 treated C57BL/6 mice
| Maximal diameter, μm | |||||||
|---|---|---|---|---|---|---|---|
| Young Isotype |
Young anti- CD3 |
Old Isotype |
Old anti- CD3 |
Age effect p value |
Treatment effect p value |
Interaction p value |
|
| 204 ± 25, 18 | 216 ± 31, 19 | 213 ± 34, 17 | 223 ± 28, 23 | 0.287 | 0.137 | 0.905 | |
| Acetylcholine Responses | |||||||
| Pretreatment | Initial tone, % | ||||||
| None | 52 ± 16, 9 | 47 ± 20, 6 | 44 ± 19, 10 | 46 ± 18, 13 | 0.514 | 0.828 | 0.580 |
| L-NAME | 58 ± 18, 9 | 54 ± 15, 6 | 50 ± 20, 15 | 48 ± 22, 12 | 0.392 | 0.715 | 0.927 |
| TEMPOL | 54 ± 17, 10 | 50 ± 13, 12 | 48 ± 16, 7 | 54 ± 12, 9 | 0.854 | 0.840 | 0.296 |
| TEMPOL & L-NAME | 63 ± 15, 10 | 56 ± 12, 11 | 60 ± 19, 7 | 56 ± 17, 8 | 0.701 | 0.116 | 0.408 |
| Sodium Nitroprusside Responses | |||||||
| Initial tone, % | |||||||
| None | 62 ± 13, 10 | 65 ± 15, 7 | 41 ± 9, 9 | 45 ± 17, 16 | 0.001 | 0.527 | 0.956 |
Data are presented as mean ± standard deviation, n. Two-way ANOVA was employed to assess the effects of age and anti-CD3 treatment and interaction. n represents the number of independent animals in each group.
Figure 7: T cell depletion results in augmented endothelium dependent dilation in mesenteric arteries from old mice.
Endothelium dependent dilation was assessed in 2nd order mesenteric arteries from (A) young Isotype control, young anti-CD3, (B) old isotype control and old anti-CD3 mice in response to increasing doses of acetylcholine. (C) Endothelium independent dilation was assessed in 2nd order mesenteric arteries in from young Isotype control, young anti-CD3, old isotype control and old anti-CD3 mice in response to increasing doses of sodium nitroprusside. Dose response curve data are expressed as means ± standard deviation. A repeated measures ANOVA was used to assess the effect of dose, treatment and dose x treatment interaction, with p values inset on each panel. n sizes are in parentheses next to the corresponding group in each panel legend and represents the number of independent animals in each group.
We next sought to determine whether the improvements in endothelium dependent dilation observed in old anti-CD3 treated mice were dependent on NO. Endothelium dependent dilation in the presence of the NO synthase inhibitor, L-NAME, was blunted in old anti-CD3 treated mice compared to dilation in the absence of L-NAME; whereas L-NAME did not alter endothelium dependent dilation in old isotype controls (Figure 8A). These observations indicate that old anti-CD3 treated mice exhibit greater NO bioavailability compared to old isotype controls. In young isotype control mice, L-NAME blunted dilation; however, although an overall effect of L-NAME was not observed in young anti-CD3 treated mice, following the observation of a significant dose x group interaction, post hoc tests indicated that L-NAME blunted dilation at the 10−4 M dose (p = 0.032, compared to in the absence of L-NAME) in young anti-CD3 treated arteries (Figure 8D). To determine whether T cells contribute to arteriolar superoxide, we assessed endothelium dependent dilation in the presence of the superoxide scavenger TEMPOL. TEMPOL improved endothelium dependent dilation in arteries from old isotype controls, but not old anti-CD3 treated mice (Figure 8B). TEMPOL did not alter dilation in arteries from young animals (Figure 8E). To determine whether the scavenging of superoxide improved NO bioavailability, we performed ACh dose responses in the presence of TEMPOL & L-NAME. The combination of TEMPOL & L-NAME abolished the TEMPOL induced improvements in dilation in arteries from the old isotype control mice (Figure 8C). The combination of TEMPOL & L-NAME did not alter endothelium dependent dilation in arteries from young mice (Figure 8F). No group differences in initial tone were observed prior to dose responses with L-NAME, TEMPOL or L-NAME & TEMPOL (Table 2).
Figure 8: T cell depletion results in augmented nitric oxide bioavailability in mesenteric arteries from old mice.
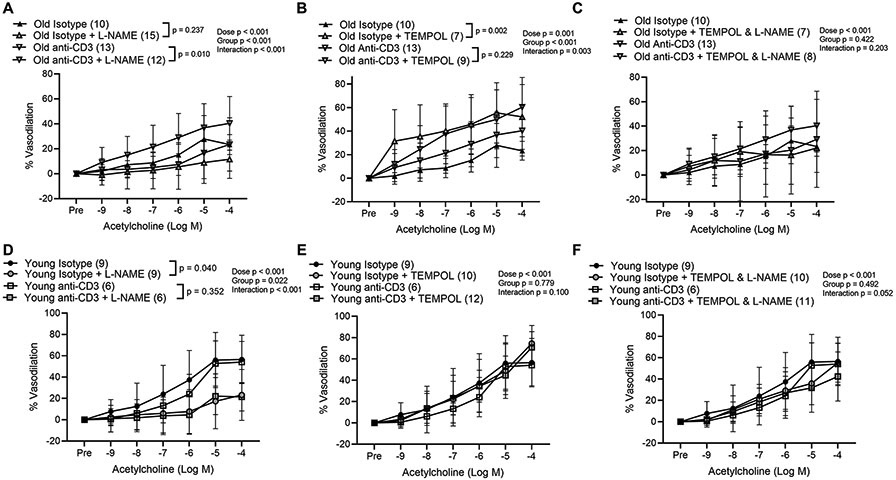
Endothelium dependent dilation in response to increasing doses of acetylcholine was assessed in 2nd order mesenteric arteries from old isotype control or old mice treated with anti-CD3 F(ab)’2 fragments in the presence or absence of (A) L-NAME, (B) TEMPOL and (C) TEMPOL & L-NAME. Endothelium dependent dilation was also assessed in arteries from young isotype control mice or young mice treated with anti-CD3 F(ab)’2 fragments in the presence or absence of (D) L-NAME, (E) TEMPOL and (F) TEMPOL & L-NAME. Dose response curve data are expressed as means ± standard deviation. A repeated measures ANOVA was used to assess the effect of dose, group and dose x group interaction, with p values inset on each panel. In the case of a significant group effect, Tukey’s post hoc test was used to compare groups with comparison p values indicated on the legend of each figure panel. In the case of a significant dose x group interaction, Tukey’s post hoc test was employed to assess significant main effects of dose with p values reported in the text. n sizes are in parentheses next to the corresponding group in each panel legend and represents the number of independent animals in each group.
Arterial Function in mice with genetic deletion of lymphocytes
We also assessed arterial function in B6.129S7-Rag1tm1Mom/J (Rag-1−/−) mice, which lack recombinase activating gene and cannot generate T or B cells, and compared these measures to age-matched C57BL/6 wild type mice. We first assessed PWV in Rag-1−/− mice and wild type mice every 3 months from 3 months up to 24 months of age. PWV increased with age in both strains but this increase was blunted in Rag-1−/− mice compared to wild type mice (Figure 9A). We next assessed endothelium dependent dilation in 2nd order mesenteric arteries from young (4-6 months) and old (22-24 months) C57BL/6 and Rag-1−/− mice. Artery diameter was greater with age but there was no strain difference. (Table 3). Arteries from Rag-1−/− mice exhibited blunted preconstriction compared to wild type (Table 3). In young mice, there were no strain differences evident in endothelium dependent dilation (Figure 9B). When comparing old wild type and old Rag-1−/− mice, we did not observe a significant group difference but there was a significant group x dose interaction and post hoc tests revealed significantly greater dilation in arteries from old Rag-1−/− mice at the 10−5 M (p = 0.004 vs. old wild type) and 10−4 M (p = 0.003 vs. old wild type) doses of ACh (Figure 9C). To assess NO bioavailability, we performed ACh dose responses in the presence of L-NAME. We found that L-NAME blunted dilation in arteries from both young and old Rag-1−/− mice (Figure 9D), indicating preserved NO bioavailability with age. Endothelium independent dilation was not affected by age or strain (Figure 8E).
Figure 9: Arterial function is preserved in a genetic model of lymphocyte deficiency.
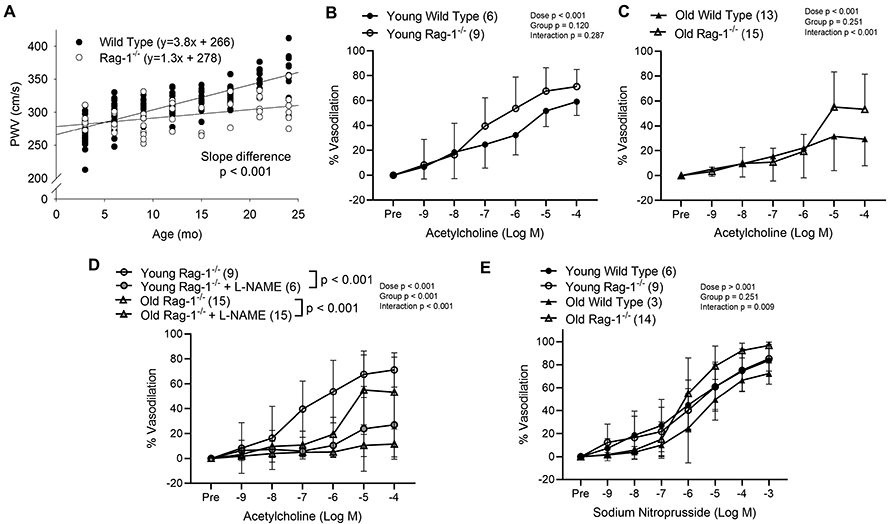
(A) Aortic pulse wave velocity (PWV) was assessed every three months in wild type and Rag-1−/− mice (n = 4-24 mice per age, PWV was assessed in some mice of both strains at multiple ages). Endothelium dependent dilation in response to increasing doses of acetylcholine was assessed in 2nd order mesenteric arteries from (B) young wild type (WT) and Rag-1−/− and (C) old WT and Rag-1−/− mice. (D) Endothelium dependent dilation in response to increasing doses of acetylcholine was assessed in 2nd order mesenteric arteries from young and old Rag-1−/− mice in the presence and absence of L-NAME. (E) Endothelium independent dilation was assessed in 2nd order mesenteric arteries from young WT and Rag-1−/− and old WT and Rag-1−/− in response to increasing doses of sodium nitroprusside. Dose response curve data are expressed as means ± SD. A repeated measures ANOVA was used to assess the effect of dose, group and dose x group interaction, with p values inset on each panel. In the case of a significant group effect, Tukey’s post hoc test was used to compare groups with comparison p values indicated on the group names on figure panel. In the case of a significant dose x group interaction, Sidak’s (2 groups) or Tukey’s post hoc test (more than 2 groups) was employed to assess significant main effects of dose with p values reported in the text. n sizes are in parentheses next to the corresponding group in each panel for panels B-E, in these panels, n represents the number of independent animals in each group.
Table 3.
Characteristics of mesenteric arteries from young and old Wild type and Rag-1−/− mice
| Maximal diameter, μm | |||||||
|---|---|---|---|---|---|---|---|
| Young Wild type |
Young Rag-1−/− |
Old Wild Type |
Old Rag-1−/− | Age effect p value |
Strain effect p value |
Interaction p value |
|
| 190 ± 23, 6 | 209 ± 34, 9 | 224 ± 38, 13 | 225 ± 36, 15 | 0.034 | 0.387 | 0.429 | |
| Acetylcholine Responses | |||||||
| Pretreatment | Initial tone, % | ||||||
| None | 62 ± 14, 6 | 47 ± 16, 9 | 59 ± 11, 13 | 58 ± 3, 15 | 0.216 | 0.031 | 0.041 |
| L-NAME | -- | 55 ± 21, 6 | -- | 64 ± 2, 15 | 0.200 | 0.622 | 0.548 |
| Sodium Nitroprusside Responses | |||||||
| Initial tone, % | |||||||
| None | 66 ± 13, 6 | 56 ± 16, 9 | 55 ± 25, 3 | 65 ± 3, 14 | 0.845 | 0.887 | 0.064 |
Data are presented as mean ± standard deviation, n. Two-way ANOVA was employed to assess the effects of age and anti-CD3 treatment and interaction. n represents the number of independent animals in each group.
DISCUSSION:
The principal findings of this investigation are as follows: 1) Aging results in increased T cell accumulation in both the aorta and mesenteric vasculature. 2) Aging results in greater numbers of proinflammatory cytokine producing T cells in both the aorta and mesenteric vasculature. 3) Anti-CD3 treatment results in T cell depletion in the blood and spleen as well as aortic and mesenteric vascular arcade T cell depletion. 4) T cell depletion decreased age-related large artery stiffness in old mice. 5) Old mice treated with anti-CD3 fragments exhibited greater endothelium dependent dilation and NO bioavailability compared to old controls. 6) Rag-1−/− mice, which lack lymphocytes, exhibited blunted increases in large artery stiffness over the lifespan as well as greater endothelium dependent dilation at old age compared to wild type mice. The observations that aging is associated with greater CD8 proinflammatory arterial T cell accumulation, that depletion of T cells improve arterial function in old mice, and that mice that lack T cells exhibit enhanced arterial function with age collectively suggest that T cells directly act as a mediator of cell non-autonomous arterial aging.
We assessed T cell accumulation and phenotype in both the aorta and mesenteric vascular arcade of old mice. We have previously observed increased T cell accumulation in the perivascular space of both the aorta and mesenteric vascular arcade (Lesniewski et al., 2011; Trott et al., 2018). In the present investigation, we extend this observation by examining T cell phenotype. We observed an inverted CD4:CD8 ratio in blood and spleen, consistent with others observations in both rodents and humans (Callahan et al., 1993; Strindhall et al., 2013). In older adults, inverted CD4:CD8 ratios are associated with mortality (Wikby et al., 1998). In this investigation, we found an inverted CD4:CD8 with age in the aorta and mesentery. Notably, CD8 cells, in particular effector memory cells, accounted for much of the age-related increase in arterial T cell accumulation in both aorta and mesentery. CD8 Effector memory cells accumulate with age and inappropriately produce inflammatory mediators (Goronzy & Weyand, 2019). CD8 T cells are also the critical subset of T cells in angiotensin II-induced hypertension in young mice (Trott et al., 2014). Combined, these observations suggest that CD8 T cells contribute to age-related arterial dysfunction, more so than CD4 T cells.
In addition to a greater number of total and CD8 cells in both the aorta and mesenteric arcade, we also found greater numbers of CD8 T cells that produce the inflammatory cytokines IFN-γ and TNF-α in the aorta, and greater numbers of CD4 and CD8 cells that produce both IFN-γ and TNF-α in the mesenteric vasculature. In the aorta, greater inflammatory cell numbers are partially due to greater aortic T cell numbers and partially due to a larger proportion of cells that produce inflammatory cytokines. In contrast, in the mesenteric vasculature, aging did not alter the phenotype of accumulating T cells and total T cell trafficking appears to explain the increased numbers of IFN-γ and TNF-α producing cells. Other investigations have established that thoracic aortic perivascular adipose (examined in this investigation) exhibits a brown adipose tissue phenotype; whereas, the abdominal aorta and mesenteric perivascular adipose tissue exhibits a white adipose phenotype (Padilla et al., 2013; Restini et al., 2018; Watts et al., 2020). Whether these differences in adipose phenotype underlie the different age-related phenotypes of T cells accumulating around the aorta and within the mesenteric arcade, and whether brown and white adipose interact to regulate arterial immune cell accumulation with advanced age is unknown and warrants future investigation.
Whether T cells from old animals exhibit intrinsic defects in trafficking and inappropriately home to the artery, or whether the old artery actively recruits these cells is largely unknown. We and others have shown that arteries from old mice and humans exhibit greater production of CCL2 and CXCL10, both important T cell recruiting chemokines (Wang et al., 2007; Donato et al., 2008; Wang et al., 2011; Song et al., 2012; Trott et al., 2017). In addition, fat associated lymphoid clusters (FALCs) develop in the mesenteric and visceral adipose with age (Lumeng et al., 2011; Camell et al., 2019). These FALCs contain large numbers of T cells, so it is possible that aging results in expansion of lymphoid tissue and/or breakdown of lymphoid capsules and release of immune cells in the perivascular adipose. In this study, we found that both young and old anti-CD3 treated mice exhibited lower mesenteric adipose mass compared to controls. This observation suggests that the relationship between T cell accumulation and adipose mass may be bidirectional. Overall, the mechanisms driving age-related arterial T cell accumulation and in particular, whether this accumulation is primarily driven by the artery, the perivascular adipose or the T cells themselves is yet to be elucidated.
In light of our previous observations that T cell accumulation is greater in old arteries compared to young (Lesniewski et al., 2011; Trott et al., 2018) we sought to determine whether T cells play a direct role in age-related arterial dysfunction. To accomplish this we employed an anti-CD3 treatment previously used to deplete T cells in the spleen (Hirsch et al., 1990), as well as in the visceral adipose (Winer et al., 2009). In the present investigation, similar to others, we found that treatment with anti-CD3 F(ab’)2 fragments depleted T cells in the spleen. In the aorta, anti-CD3 treatment resulted in blunted total, CD4, and CD8 numbers in both young and old mice. In the mesenteric vascular arcade, anti-CD3 treatment resulted in lower total T cell numbers. We also sought to determine whether anti-CD3 treatment might alter recruitment and retention of arterial B cells and macrophages, two cell types that have also been shown to contribute to arterial dysfunction in acute hypertension models (Wenzel et al., 2011; Chan et al., 2015). We found that age, but not anti-CD3 treatment, altered B cell and macrophage numbers in both the aorta and mesenteric vasculature. These results indicate that our T cell depletion regimen was effective in the spleen, blood, and arteries. These data also indicate that T cells do not play a critical role in recruitment or retention of other immune cell types to the aged artery.
Increased arterial stiffness that occurs with age (Vaitkevicius et al., 1993; Lakatta & Levy, 2003) contributes to increased pulse pressure, systolic blood pressure and to the subsequent increased risk for cardiovascular disease, in particular cerebrovascular disease and heart failure (Shirwany & Zou, 2010; Ben-Shlomo et al., 2014). In the present investigation, we found that large artery stiffness is greater with advanced age and that depletion of T cells significantly ameliorated age-related arterial stiffening. Consistent with our finding that aging results in greater numbers of aortic TNF-α and IFN-γ producing T cells, there is evidence that both cytokines play a role in aortic stiffness. TNF-α antagonists consistently lower large artery stiffness in rheumatoid arthritis patients (Vlachopoulos et al., 2018) and TNF-α derived from the perivascular adipose has been shown to mediate aortic stiffening in a rodent model of metabolic syndrome (DeVallance et al., 2018). T cell derived IFN-γ has been shown to mediate increased aortic stiffening in a mouse model of transplant rejection (Zhou et al., 2015) and IFN-γ mediates end organ (including aortic) damage in Angiotensin II treated mice (Pan et al., 2020). Exercise, which lowers age-related large artery stiffening in both rodents and humans (Vaitkevicius et al., 1993; Fleenor et al., 2010), also ameliorates age-related increases in aortic TNF-α and IFN-γ (Lesniewski et al., 2011). Combined with our findings in the current investigation, these data suggest that T cell derived TNF-α and IFN-γ play a critical role in the development of large artery stiffness with age.
To gain insight into how T cells and pro-inflammatory cytokines mediate increased aortic stiffening we examined aortic gene expression for enzymes responsible for reactive oxygen species production and scavenging. Arterial reactive oxygen species, in particular superoxide, have been shown to play a major role in age-related aortic stiffness (Fleenor et al., 2012; Gioscia-Ryan et al., 2018). We found that aging resulted in greater aortic Nox2 gene expression that was not altered by anti-CD3 treatment. We also found that aortic gene expression for the cytosolic (Sod1), mitochondrial (Sod2) and extracellular (Sod3) isoforms of superoxide dismutase were greater in mice treated with anti-CD3 fragments. This observation suggests that proinflammatory T cells restrain antioxidant gene expression in the arterial wall. Literature suggests that TNF-α blunts, but IFN-γ enhances Sod3 expression in cultured vascular smooth muscle cells (Strålin & Marklund, 2000). Furthermore, IFN-γ directly impairs endothelium dependent relaxation in young mouse aortic rings via increases in arterial superoxide (Mikolajczyk et al., 2016). Middle aged (9 month) p22phox transgenic mice that generate increased arterial reactive oxygen species, demonstrate increased large artery stiffness and aortic collagen deposition, and these processes are dependent on the presence of arterial T cells (Wu et al., 2016). Collectively, these data support the concept that proinflammatory T cells of the artery play an important role in the regulation of reactive oxygen species and age-related increases in aortic stiffness.
Aortic stiffness is regulated by both biochemical processes (i.e. a balance of reactive oxygen species and NO) and mechanical properties of the aorta driven by a balance of structural proteins such as collagen and elastin. Further, there is interplay between these biochemical and mechanical factors that govern overall aortic stiffness (Lakatta & Levy, 2003; Wang et al., 2020). A limitation of the present study is that we did not complement our in vivo aortic stiffness measures with ex vivo assessments of the elastic modulus of the aorta or with assessment of collagen and elastin protein content so we cannot precisely delineate the mechanisms by which T cells contribute to aortic stiffness with advancing age.
In addition to improvements in large artery stiffness with anti-CD3 treatment, we observed that old anti-CD3 treated mice also exhibited greater endothelium dependent dilation in the mesenteric arteries compared to old controls. This has systemic impact as the relative state of vascular tone in the mesenteric arteries is a determining factor in total peripheral resistance and, therefore can influence blood pressure. The improvements in mesenteric artery endothelium dependent dilation with T cell depletion appear to be due to greater NO bioavailability mediated by lower arteriolar superoxide. T cell derived cytokines have been shown to blunt endothelium dependent dilation through reactive oxygen species (Zhang et al., 2006), and our group has previously shown that TNF-α directly impairs NO bioavailability in visceral adipose arteries from young mice (Donato et al., 2012). In addition, T cell derived IFN-γ impairs endothelium dependent dilation in the skeletal muscle microcirculation of hypercholesterolemic mice (Stokes et al., 2007). Together these data suggest that chronic IFN-γ and TNF-α release from T cells in the old mesenteric vascular arcade contributes to age-related impairments in NO bioavailability and endothelial function.
Finally, we observed that Rag-1−/− mice, which lack lymphocytes due to genetic impairments in T cell receptor and antibody recombination, exhibit blunted increases in aortic stiffness from 3-24 months of age and preserved endothelium dependent dilation in mesenteric arteries with age in comparison to wild type mice. Similarly, when crossed with Rag-1−/− mice, p22phox transgenic mice, which generate greater arterial reactive oxygen species, exhibited blunted increases in aortic stiffness from age 3-9 months compared to mice on a wild type background (Wu et al., 2016). This observation supports the concept that there is a bi-directional relationship between T cells and arterial reactive oxygen species. It should be noted that Rag-1−/− mice lack both T and B cells and so a role for the absence of B cells in the preservation of arterial function in old Rag-1−/− mice cannot be completely ruled out. To provide insight into the potential role of other immune cell types, we observed that aging resulted in greater aortic and mesenteric B cell and macrophage accumulation in wild type mice in agreement with our previous findings (Trott et al., 2018). In anti-CD3 treated wild type mice we found no alterations in aortic and mesenteric B cell or macrophage accumulation indicating that improvements in arterial function are not dependent on B cells or macrophages. These observations underscore the concept that T cells per se play a critical role in arterial aging.
Complementary to our findings, there is indirect evidence in humans that aging T cells play a role in arterial dysfunction. Patients with rheumatoid arthritis, a T cell mediated autoimmune disease, exhibit increased risk for cardiovascular disease independent of traditional risk factors (del Rincon et al., 2001). In addition, hypertension (Youn et al., 2013) and large artery stiffness (Yu et al., 2017) are associated with increased circulating pro-inflammatory CD8+ T cells with a phenotype consistent with aged T cells. Global T cell depletion, as employed in this study, is not feasible as a treatment strategy for cardiovascular disease due to increased risk of infectious disease in older adults. However, results from the CANTOS trial provide initial proof-of-concept that targeting inflammation, specifically IL-1β, can reduce cardiovascular events (Ridker et al., 2017). Identifying the subtype of T cells responsible for arterial dysfunction, the mechanisms by which these cells are recruited to the artery, and the mechanisms by which T cells interact with the various cell types of the artery (i.e. endothelial, smooth muscle, fibroblast, perivascular adipose) all have potential to lead to novel therapeutic targets to preserve cardiovascular health in the elderly.
In summary, in this report we show that aging results in increased accumulation of IFN-γ and TNF-α producing T cells in both the aorta and mesenteric vasculature. Anti-CD3 treatment resulted in blunted T cell numbers in both aorta and mesentery, reversed large artery stiffness and resulted in greater endothelium dependent dilation in old mice. In concert with the data from anti-CD3 treated mice, we found that mice with lifelong genetic deletion of lymphocytes exhibited blunted increases in large artery stiffness and preserved mesenteric arteriolar endothelium dependent dilation with age. This investigation provides evidence that T cells can mediate arterial dysfunction in absence of supraphysiological stimuli (i.e. Angiotensin II) or genetic models (i.e. deletion of ApoE or Ldlr) of atherosclerosis. Collectively, these results indicate that T cells are major contributors to both large elastic artery and resistance arteriolar dysfunction with age.
Supplementary Material
KEY POINTS:
Increased large artery stiffness and impaired endothelium dependent dilation occur with advanced age.
We sought to determine whether T cells mechanistically contribute to age-related arterial dysfunction.
We found that old mice exhibited greater proinflammatory T cell accumulation around both the aorta and mesenteric arteries.
Pharmacologic depletion or genetic deletion of T cells in old mice resulted in ameliorated large artery stiffness and greater endothelium dependent dilation compared to mice with T cells intact.
Acknowledgements:
Flow Cytometry work was supported by the University of Utah Flow Cytometry Facility, National Cancer Institute award number 5P30CA042014-24 and National Center for Research Resources award number 1S10RR026802-01.
Funding:
This work was supported by National Institutes of Health grants: K01 AG061271 (DWT), R01 AG060395 (AJD), R01 AG050238 (AJD), R01 AG048366 (LAL) Veteran's Affairs Merit Review Award I01 BX004492 (LAL) from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The contents do not represent the views of the U.S. Department of Veterans Affairs, the National Institutes of Health or the United States Government. SIB was supported by 5T32HL139451-02.
Footnotes
Competing Interests: None.
Data availability statement:
All data are presented as individual data points where feasible. Data not presented with individual data points is available as supporting information online.
REFERENCES:
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR & Wilkinson IB (2014). Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K & Katusic ZS (2004). Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol 287, H2448–2453. [DOI] [PubMed] [Google Scholar]
- Callahan JE, Kappler JW & Marrack P (1993). Unexpected expansions of CD8-bearing cells in old mice. J Immunol 151, 6657–6669. [PubMed] [Google Scholar]
- Camell CD, Günther P, Lee A, Goldberg EL, Spadaro O, Youm YH, Bartke A, Hubbard GB, Ikeno Y, Ruddle NH, Schultze J & Dixit VD (2019). Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell Metab 30, 1024–1039.e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, Kim HA, Krishnan SM, Lewis CV, Salimova E, Tipping P, Vinh A, Samuel CS, Peter K, Guzik TJ, Kyaw TS, Toh BH, Bobik A & Drummond GR (2015). Obligatory Role for B Cells in the Development of Angiotensin II-Dependent Hypertension. Hypertension 66, 1023–1033. [DOI] [PubMed] [Google Scholar]
- De Miguel C, Das S, Lund H & Mattson DL (2010). T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298, R1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rincon ID, Williams K, Stern MP, Freeman GL & Escalante A (2001). High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 44, 2737–2745. [DOI] [PubMed] [Google Scholar]
- DeVallance E, Branyan KW, Lemaster K, Olfert IM, Smith DM, Pistilli EE, Frisbee JC & Chantler PD (2018). Aortic dysfunction in metabolic syndrome mediated by perivascular adipose tissue TNFα- and NOX2-dependent pathway. Exp Physiol 103, 590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Black AD, Jablonski KL, Gano LB & Seals DR (2008). Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7, 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE & Seals DR (2007). Direct Evidence of Endothelial Oxidative Stress With Aging in Humans Relation to Impaired Endothelium-Dependent Dilation and Upregulation of Nuclear Factor-κB. Circ Res 100, 1659–1666. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Henson GD, Morgan RG, Enz RA, Walker AE & Lesniewski LA (2012). TNF-α impairs endothelial function in adipose tissue resistance arteries of mice with diet-induced obesity. Am J Physiol Heart Circ Physiol 303, H672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA & Seals DR (2013). Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 12, 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ & Lesniewski LA (2009). Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587, 3271–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhage R, Gourdy P, Brouchet L, Jawien J, Fouque MJ, Fiévet C, Huc X, Barreira Y, Couloumiers JC, Arnal JF & Bayard F (2004). Deleting TCR alpha beta+ or CD4+ T lymphocytes leads to opposite effects on site-specific atherosclerosis in female apolipoprotein E-deficient mice. Am J Pathol 165, 2013–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch RM, Vergona R, Sullivan ME & Wang YX (2001). Nitric oxide synthase inhibition increases aortic stiffness measured by pulse wave velocity in rats. Cardiovasc Res 51, 351–358. [DOI] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA & Seals DR (2010). Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588, 3971–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Seals DR, Zigler ML & Sindler AL (2012). Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard M, Roddy MA, Creager SJ & Creager MA (1996). Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 27, 849–853. [DOI] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP & Seals DR (2018). Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol (1985) 124, 1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ & Weyand CM (2019). Mechanisms underlying T cell ageing. Nat Rev Immunol 19, 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros R, Van Wert R, You X, Thorin E & Husain M (2002). Effects of age, gender, and blood pressure on myogenic responses of mesenteric arteries from C57BL/6 mice. Am J Physiol Heart Circ Physiol 282, H380–388. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C & Harrison DG (2007). Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204, 2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch R, Bluestone JA, DeNenno L & Gress RE (1990). Anti-CD3 F(ab')2 fragments are immunosuppressive in vivo without evoking either the strong humoral response or morbidity associated with whole mAb. Transplantation 49, 1117–1123. [DOI] [PubMed] [Google Scholar]
- Lakatta EG & Levy D (2003a). Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises Part I: Aging arteries: A "set up" for vascular disease. Circulation 107, 139–146. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ & Seals DR (2009). B6D2F1 Mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. The journals of gerontology. Series A, Biological sciences and medical sciences 64, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ & Seals DR (2011). Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301, H1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M & Heiss G (1999). Arterial stiffness and the development of hypertension. The ARIC study. Hypertension 34, 201–206. [DOI] [PubMed] [Google Scholar]
- Livak KJ & Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Liu J, Geletka L, Delaney C, Delproposto J, Desai A, Oatmen K, Martinez-Santibanez G, Julius A, Garg S & Yung RL (2011). Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol 187, 6208–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin DR, Auduong Y, Gogulamudi VR, Liu Y, Islam MT, Lesniewski LA & Donato AJ (2020). Lifelong SIRT-1 overexpression attenuates large artery stiffening with advancing age. Aging (Albany NY) 12, 11314–11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk TP, Nosalski R, Szczepaniak P, Budzyn K, Osmenda G, Skiba D, Sagan A, Wu J, Vinh A, Marvar PJ, Guzik B, Podolec J, Drummond G, Lob HE, Harrison DG & Guzik TJ (2016). Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. Faseb J 30, 1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, Richardson RS & Donato AJ (2013). Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am J Physiol Heart Circ Physiol 305, H251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW & Delp MD (2002). Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arteries. Am J Physiol Heart Circ Physiol 283, H1662–1672. [DOI] [PubMed] [Google Scholar]
- Padilla J, Jenkins NT, Vieira-Potter VJ & Laughlin MH (2013). Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol 304, R543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan XX, Wu F, Chen XH, Chen DR, Chen HJ, Kong LR, Ruan CC & Gao PJ (2020). T cell senescence accelerates Angiotensin II-induced target organ damage. Cardiovasc Res. [DOI] [PubMed] [Google Scholar]
- Pierce GL, Lesniewski LA, Lawson BR, Beske SD & Seals DR (2009). Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 119, 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ras RT, Streppel MT, Draijer R & Zock PL (2013). Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol 168, 344–351. [DOI] [PubMed] [Google Scholar]
- Restini CBA, Ismail A, Kumar RK, Burnett R, Garver H, Fink GD & Watts SW (2018). Renal perivascular adipose tissue: Form and function. Vascul Pharmacol 106, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ & Group CT (2017). Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Shirwany NA & Zou MH (2010). Arterial stiffness: a brief review. Acta Pharmacol Sin 31, 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Shen H, Schenten D, Shan P, Lee PJ & Goldstein DR (2012). Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology 32, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes KY, Gurwara S & Granger DN (2007). T-Cell - Derived interferon-gamma contributes to arteriolar dysfunction during acute Hypercholesterolemia. Arteriosclerosis Thrombosis and Vascular Biology 27, 1998–2004. [DOI] [PubMed] [Google Scholar]
- Strindhall J, Skog M, Ernerudh J, Bengner M, Lofgren S, Matussek A, Nilsson BO & Wikby A (2013). The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: the Swedish HEXA immune study. Age (Dordr) 35, 985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strålin P & Marklund SL (2000). Multiple cytokines regulate the expression of extracellular superoxide dismutase in human vascular smooth muscle cells. Atherosclerosis 151, 433–441. [DOI] [PubMed] [Google Scholar]
- Trott DW, Gunduz F, Laughlin MH & Woodman CR (2009). Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 106, 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DW, Henson GD, Ho MHT, Allison SA, Lesniewski LA & Donato AJ (2018). Age-related arterial immune cell infiltration in mice is attenuated by caloric restriction or voluntary exercise. Exp Gerontol 109, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DW, Lesniewski LA & Donato AJ (2017). Selected life-extending interventions reduce arterial CXCL10 and macrophage colony-stimulating factor in aged mouse arteries. Cytokine 96, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DW, Seawright JW, Luttrell MJ & Woodman CR (2011). NAD(P)H oxidase-derived reactive oxygen species contribute to age-related impairments of endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol 110, 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y & Harrison DG (2014). Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64, 1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC & Lakatta EG (1993). Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88, 1456–1462. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Gravos A, Georgiopoulos G, Terentes-Printzios D, Ioakeimidis N, Vassilopoulos D, Stamatelopoulos K & Tousoulis D (2018). The effect of TNF-a antagonists on aortic stiffness and wave reflections: a meta-analysis. Clin Rheumatol 37, 515–526. [DOI] [PubMed] [Google Scholar]
- Wang M, Monticone RE & McGraw KR (2020). Proinflammation, profibrosis, and arterial aging. Aging Med (Milton) 3, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Spinetti G, Monticone RE, Zhang J, Wu J, Jiang L, Khazan B, Telljohann R & Lakatta EG (2011). A local proinflammatory signalling loop facilitates adverse age-associated arterial remodeling. PLoS One 6, e16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R & Lakatta EG (2007). Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 50, 219–227. [DOI] [PubMed] [Google Scholar]
- Watts SW, Flood ED, Garver H, Fink GD & Roccabianca S (2020). A New Function for Perivascular Adipose Tissue (PVAT): Assistance of Arterial Stress Relaxation. Sci Rep 10, 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A & Munzel T (2011). Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124, 1370–1381. [DOI] [PubMed] [Google Scholar]
- Wikby A, Maxson P, Olsson J, Johansson B & Ferguson FG (1998). Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev 102, 187–198. [DOI] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D & Dosch HM (2009). Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 15, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, Chen W, Mernaugh RL, Cai H, Bernstein KE, Goronzy JJ, Weyand CM, Curci JA, Barbaro NR, Moreno H, Davies SS, Roberts LJ, 2nd, Madhur MS & Harrison DG (2016). Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest 126, 50–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, Yoo OJ, Shin EC & Park S (2013). Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62, 126–133. [DOI] [PubMed] [Google Scholar]
- Yu HT, Youn JC, Kim JH, Seong YJ, Park SH, Kim HC, Lee WW, Park S & Shin EC (2017). Arterial Stiffness Is Associated With Cytomegalovirus-Specific Senescent CD8. J Am Heart Assoc 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarron BF, Mergian TA, Cho KW, Martinez-Santibanez G, Luan D, Singer K, DelProposto JL, Geletka LM, Muir LA & Lumeng CN (2017). Macrophage Proliferation Sustains Adipose Tissue Inflammation in Formerly Obese Mice. Diabetes 66, 392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CH, Hein TW, Wang W, Ren Y, Shipley RD & Kuo L (2006). Activation of JNK and xanthine oxidase by TNF-alpha impairs nitric oxide-mediated dilation of coronary arteries. Journal of Molecular and Cellular Cardiology 40, 247–257. [DOI] [PubMed] [Google Scholar]
- Zhou J, Qin LF, Yi T, Ali R, Li QL, Jiao Y, Li GX, Tobiasova Z, Huang Y, Zhang JS, Yun JJ, Sadeghi MM, Giordano FJ, Pober JS & Tellides G (2015). Interferon-gamma-Mediated Allograft Rejection Exacerbates Cardiovascular Disease of Hyperlipidemic Murine Transplant Recipients. Circulation Research 117, 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are presented as individual data points where feasible. Data not presented with individual data points is available as supporting information online.