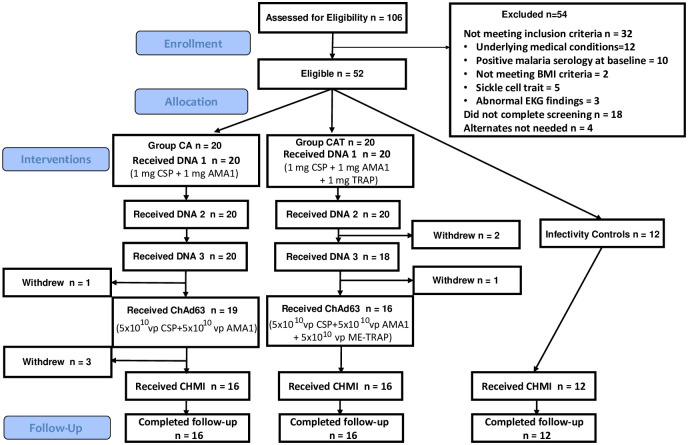
Fig 1. Flow diagram of immunized and control subjects.
Fifty-two subjects met all eligibility criteria and were randomly allocated to the CA group (n = 20), CAT group (n = 20) and infectivity controls (n = 12). CA group: Prior to the ChAd63 immunization, 1 subject withdrew for personal reasons; prior to CHMI, 3 subjects were withdrawn: 1 due to a laboratory abnormality thought possibly related to the study interventions, 1 due to relocation, and 1 due to suicidal ideation requiring inpatient admission (unrelated to the study intervention). CAT group: Prior to the third DNA immunizations, 2 subjects withdrew: 1 subject due to an unrelated SAE and 1 subject due to a medical issue (commencing latent tuberculosis treatment); prior to ChAd63 immunizations, 2 subjects withdrew, 1 subject due to pregnancy, and 1 subject due to a SAE grade 4 laboratory abnormality (neutropenia) likely attributed to benign ethnic neutropenia but possibly to study intervention.