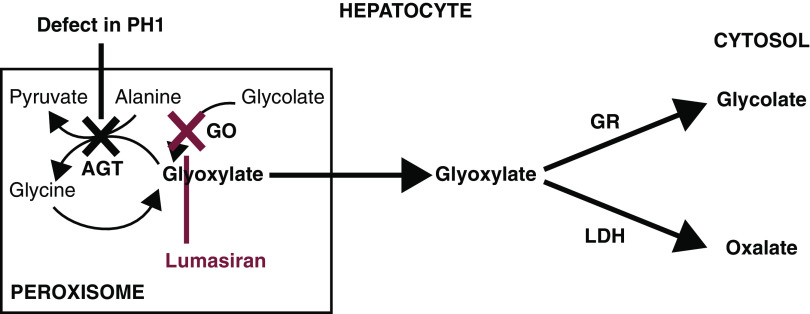
Figure 1.
Defect in glyoxylate metabolism in hepatocytes of patients with primary hyperoxaluria type 1 and lumasiran therapeutic hypothesis. Patients with primary hyperoxaluria type 1 have reduced or absent alanine-glyoxylate aminotransferase (AGT) function, resulting in increased oxalate and glycolate production by the hepatocyte, both of which are excreted by the kidneys. AGT in the liver peroxisome metabolizes glyoxylate to glycine. When AGT is deficient (black X), glyoxylate cannot be metabolized to glycine. Excess glyoxylate accumulates and is converted to oxalate. Excess hepatic oxalate is then transported to the kidney for excretion in the urine, leading to hyperoxaluria. In the kidneys, excess oxalate combines with calcium, which due to its insolubility, can readily crystallize in the urinary tract and lead to recurrent urolithiasis and nephrocalcinosis, causing progressive kidney damage. As kidney function worsens, there is a reduction in the capacity of the kidneys to clear oxalate from the blood, leading to an increase in plasma oxalate and subsequent deposition of calcium oxalate crystals in tissues and vital organs, including bone, heart, retina, and skin, recognized clinically as systemic oxalosis. Lumasiran targets liver hydroxyacid oxidase 1 mRNA (red X), decreasing production of glycolate oxidase (GO), and hence, reducing hepatic oxalate production and delivery of oxalate to the kidneys for excretion. GR, glyoxylate reductase; LDH, lactate dehydrogenase. Adapted from ref. 27, with permission.