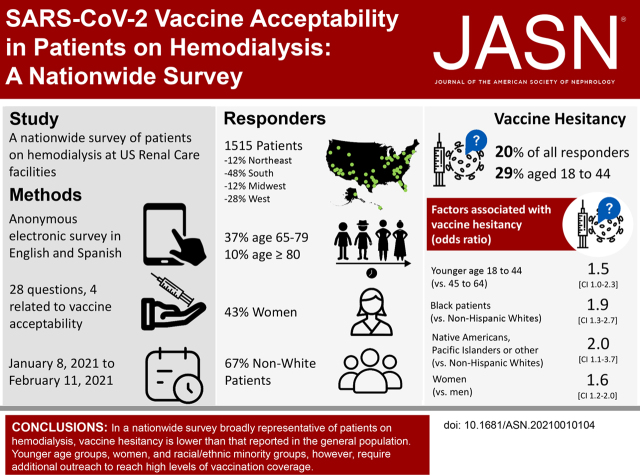
Significance Statement
High uptake of the SARS-COV-2 vaccine among patients on dialysis is critical to mitigating the devastating rates of COVID-19–related complications and deaths observed in the dialysis population. In a nationwide vaccine acceptability survey involving 150 dialysis facilities in the United States and broadly representative of this patient population, the authors found that, overall, one in five patients had vaccine hesitancy, as did one in four Black patients or patients aged 18–44 years. One in three responders identified dialysis staff as key sources of information about COVID-19 vaccines. Patients on hemodialysis who were vaccine hesitant were chiefly concerned about side effects. These findings highlight the opportunities available to dialysis networks in facilitating vaccine uptake among patients on dialysis and identify specific subgroups for which additional outreach is necessary.
Keywords: dialysis, vaccine, SARS-CoV-2, ESKD, COVID-19
Visual Abstract
Abstract
Background
Patients on dialysis are at increased risk for COVID-19–related complications. However, a substantial fraction of patients on dialysis belong to groups more likely to be hesitant about vaccination.
Methods
With the goal of identifying strategies to increase COVID-19 vaccine uptake among patients on hemodialysis, we conducted a nationwide vaccine acceptability survey, partnering with a dialysis network to distribute an anonymized English and Spanish language online survey in 150 randomly selected facilities in the United States. We used logistic regression to evaluate characteristics of vaccine-hesitant persons.
Results
A total of 1515 (14% of eligible) patients responded; 20% of all responders, 29% of patients aged 18–44 years, and 29% of Black responders reported being hesitant to seek the COVID-19 vaccine, even if the vaccine was considered safe for the general population. Odds of vaccine hesitancy were higher among patients aged 18–44 years versus those 45–64 years (odds ratio [OR], 1.5; 95% confidence interval [95% CI], 1.0 to 2.3), Black patients versus non-Hispanic White patients (OR, 1.9; 95% CI, 1.3 to 2.7), Native Americans or Pacific Islanders versus non-Hispanic White patients (OR, 2.0; 95% CI, 1.1 to 3.7), and women versus men (OR, 1.6; 95% CI, 1.2 to 2.0). About half (53%) of patients who were vaccine hesitant expressed concerns about side effects. Responders’ main information sources about COVID-19 vaccines were television news and dialysis staff (68% and 38%, respectively).
Conclusions
A substantial proportion of patients receiving in-center hemodialysis in the United States are hesitant about seeking COVID-19 vaccination. Facilitating uptake requires outreach to younger patients, women, and Black, Native American, or Pacific Islander patients, and addressing concerns about side effects.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/JASN/2021_07_07_JASN2021010104.mp3
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection results in high rates of hospitalization and death among patients receiving dialysis. Medicare data tracking coronavirus disease 2019 (COVID-19) hospitalizations indicate that close to 7000 persons with ESKD are hospitalized per 100,000 beneficiaries, a rate three- to four-fold higher than in older or disabled persons.1 On hospitalization, early reported mortality rates exceeded 25%.2
Considering the serious health implications of SARS-CoV-2 infection in patients receiving dialysis, combined with the potential for increased risk for exposure with travel to, from, and during the provision of in-center hemodialysis or crosstraffic between dialysis facilities and skilled nursing facilities,3 some states are prioritizing vaccination to these patients, almost half of whom are also over 65 years of age. A substantial fraction of persons receiving dialysis, however, belong to racial, ethnic, socioeconomic, cultural, and religious groups more likely to be vaccine hesitant.4–7
To inform programs and policies aimed at promoting COVID-19 vaccine uptake in this vulnerable population, we offered a nationwide survey to persons undergoing in-center hemodialysis in 150 randomly selected facilities in January 2021. Our goal was to estimate rates of vaccine hesitancy, and describe the demographic and clinical characteristics of vaccine-hesitant persons and elicit their major concerns.
Methods
We distributed an anonymized online survey in facilities of a mid-to-large–sized dialysis organization from January 8 to February 11, 2021. The study received Institutional Review Board approval from the Stanford Institution Review Board.
Sampling
Our sampling frame consisted of 331 hemodialysis facilities with ≥30 patients managed by US Renal Care. These facilities are located throughout the United States, and treat a population that is representative of the overall US dialysis population by age, sex, and race/ethnicity, although underrepresenting the Midwest (Supplemental Table 1). We randomly selected 150 facilities using implicit stratification by region and zip code, followed by systematic sampling with fractional polynomials.8 We removed from the sampling frame a single facility where we piloted the survey.
Survey
We designed a survey with 28 questions, and four major subheadings: COVID-19 and vaccine, COVID-19 effects, responder health and family structure, and demographic data (Supplemental Survey). These subheadings followed the conceptual framework recommended by the SAGE Working Group on Vaccine Hesitancy,9 with four questions related to COVID-19 vaccine hesitancy drawn from published surveys in the United States and worldwide.4,5,9–12 Demographic questions were on the basis of the US Census or National Health and Nutrition Examination Survey.
Because Spanish is the second most commonly spoken language in the United States,13 a native speaker (P.G.) created a Spanish language version of the survey. We generated the survey using Qualtrics software, Version XM (Qualtrics, Provo, UT). We estimated the time required to complete the survey to be 9 minutes. Before publishing the survey, we piloted in one facility with English and native Spanish speakers. We deployed a final version with an accompanying instructional video and slide deck sent to facility managers and social workers; social workers were designated survey “champions.” We held several progress calls with facilities to assist with survey uptake.
We distributed the surveys to all patients aged ≥18 years using one of two methods: (1) facility iPads with a link to the survey, or (2) quick response codes to access the survey via a smartphone. If participants answered at least one survey question, we included their responses.
Statistical Analyses
We expressed categorical variables as counts (percentage). We used logistic regression to calculate odds ratio (OR) and 95% confidence interval (95% CI) for correlates of vaccine hesitancy. We linked zip codes to the US census region. For the purposes of the logistic regression analysis, we defined vaccine hesitancy as answering not sure, probably not, or definitely not, to any of the four COVID-19 vaccine-related questions. In total, 93% of the responders completed all questions. Two responders missing the outcome (vaccine hesitancy) and four missing regions were removed from the logistic regression analysis. We assumed data were missing at random, conditional on observed variables and used multiple imputation through chained equations to generate eight imputed datasets.14,15 The imputation model included all independent variables and the outcome of vaccine hesitancy. We combined the estimates and SEMs obtained from the logistic regression model applied to each imputed dataset using Rubin’s rules.16
We used SAS version 9.4 (SAS Institute, Inc, Cary, NC) and Stata MP version 16.1 (StataCorp, College Station, TX) to perform the analyses.
Results and Discussion
A total of 1515 patients among 10,974 responded to the survey, a response rate of 14%. Supplemental Figure 1 depicts the locations from which surveys were returned; no responses were returned from Northwestern facilities, but otherwise responding zip codes matched the geographic distribution of sampled facilities. A majority (98%) of the responses were returned in January 2021, before widespread roll out of vaccines. As shown in Supplemental Table 1, patients responding to the survey matched the distribution of the US dialysis population by age, sex, and race/ethnicity. Patients on dialysis from the Midwest (12% of survey responders) were slightly underrepresented (19% of US patients); patients from the US South and West were slightly overrepresented.
Responders from the Northeast were older, responders from the West were more likely to be male and of Hispanic ethnicity, and responders from the South were more likely to be Black responders (Table 1). Of survey responders, 15% had a family member or close acquaintance who had died from COVID-19. Only 13% of responders did not receive, or were not planning to receive, an influenza vaccine during the 2020–21 season. In total, 65% of responders had at least one additional comorbid condition, including 14% with a history of a kidney transplant or on immunosuppressant medications.
Table 1.
Characteristics of survey responders by US Census region of residence
| Patient Characteristics | Totala n=1515 | Northeast n=177 | South n=733 | Midwest n=185 | West n=418 |
|---|---|---|---|---|---|
| Age, yrs, n (%) | |||||
| 18–44 | 149 (10) | 9 (5) | 77 (11) | 15 (8) | 48 (12) |
| 45–64 | 608 (40) | 62 (35) | 325 (44) | 65 (35) | 156 (37) |
| 65–79 | 557 (37) | 80 (45) | 243 (33) | 71 (39) | 163 (39) |
| ≥80 | 158 (10) | 24 (13) | 64 (9) | 32 (17) | 38 (9) |
| Missing | 43 (3) | 4 (2) | 24 (3) | 2 (1) | 13 (3) |
| Sex, n (%) | |||||
| Male | 806 (53) | 85 (48) | 386 (53) | 96 (52) | 239 (57) |
| Female | 647 (43) | 88 (50) | 312 (42) | 85 (46) | 162 (39) |
| Missing | 62 (4) | 4 (2) | 35 (5) | 4 (2) | 17 (4) |
| Race and ethnicity, n (%) | |||||
| Hispanic | 372 (24) | 12 (7) | 183 (25) | 11 (6) | 166 (40) |
| Non-Hispanic White | 448 (30) | 94 (53) | 179 (24) | 113 (61) | 62 (15) |
| Non-Hispanic Black | 456 (30) | 57 (32) | 304 (42) | 50 (27) | 45 (11) |
| Asian | 119 (8) | 8 (5) | 15 (2) | 2 (1) | 94 (22) |
| Otherb | 77 (5) | 4 (2) | 28 (4) | 6 (3) | 39 (9) |
| Missing | 43 (3) | 2 (1) | 24 (3) | 3 (2) | 12 (3) |
| ZCTA poverty, n (%) | |||||
| <10% | 431 (29) | 35 (20) | 188 (26) | 65 (35) | 143 (34) |
| 10% to <20% | 549 (36) | 137 (77) | 216 (30) | 25 (14) | 171 (41) |
| 20%to <30% | 259 (17) | 4 (2) | 183 (25) | 59 (32) | 13 (3) |
| ≥30% | 239 (16) | 0 (0) | 113 (15) | 36 (19) | 90 (22) |
| Missing | 37 (3) | 1 (1) | 33 (5) | 0 (0) | 0 (0) |
| Level of education, n (%) | |||||
| <9th grade | 144 (10) | 10 (6) | 68 (9) | 10 (5) | 56 (13) |
| 9–11th grade | 154 (10) | 22 (12) | 78 (11) | 21 (11) | 33 (8) |
| High school | 548 (36) | 62 (35) | 282 (39) | 89 (48) | 115 (28) |
| College | 626 (41) | 81 (46) | 281 (38) | 63 (34) | 201 (48) |
| Missing | 43 (3) | 2 (1) | 24 (3) | 2 (1) | 13 (3) |
| Family member or close acquaintancec with COVID-19, n (%) | |||||
| Yes | 576 (38) | 68 (39) | 292 (40) | 84 (45) | 132 (31) |
| No | 893 (59) | 105 (59) | 420 (57) | 97 (52) | 271 (65) |
| Missing | 46 (3) | 4 (2) | 21 (3) | 4 (2) | 15 (4) |
| Family member or close acquaintancec died from COVID-19, n (%) | |||||
| Yes | 229 (15) | 26 (14) | 114 (16) | 37 (20) | 51 (12) |
| No | 1246 (82) | 150 (85) | 597 (81) | 146 (79) | 353 (85) |
| Missing | 40 (3) | 1 (1) | 22 (3) | 2 (1) | 14 (3) |
| Multigenerational household, n (%) | |||||
| Yes | 506 (33) | 49 (28) | 242 (33) | 62 (34) | 152 (36) |
| No | 971 (64) | 125 (71) | 471 (64) | 121 (65) | 254 (61) |
| Missing | 38 (3) | 3 (1) | 20 (3) | 2 (1) | 12 (3) |
| Got the influenza vaccine or planning to get the influenza vaccine, n (%) | |||||
| Yes | 1285 (85) | 137 (77) | 626 (85) | 152 (82) | 369 (88) |
| Nod | 191 (13) | 39 (22) | 86 (12) | 31 (17) | 35 (8) |
| Missing | 39 (2) | 1 (1) | 21 (3) | 2 (1) | 14 (4) |
ZCTA, zip code tabulation area.
Including those from an unknown location.
A majority (64%) were Native Americans or Pacific Islanders.
Close acquaintance defined as someone who the respondent interacts with weekly.
Includes those who said “Have not decided.”
Among responders, 20% were reluctant to seek the COVID-19 vaccine even if the vaccine was considered safe for the general population (Figure 1), with younger age groups and Black responders more likely to indicate hesitancy (29% in responders aged 18%–44% and 29% in Black responders). If the vaccine was offered at the dialysis facility, vaccine hesitancy was slightly lower overall (18%) and in both groups (25% in responders aged 18%–44% and 26% in Black responders).
Figure 1.
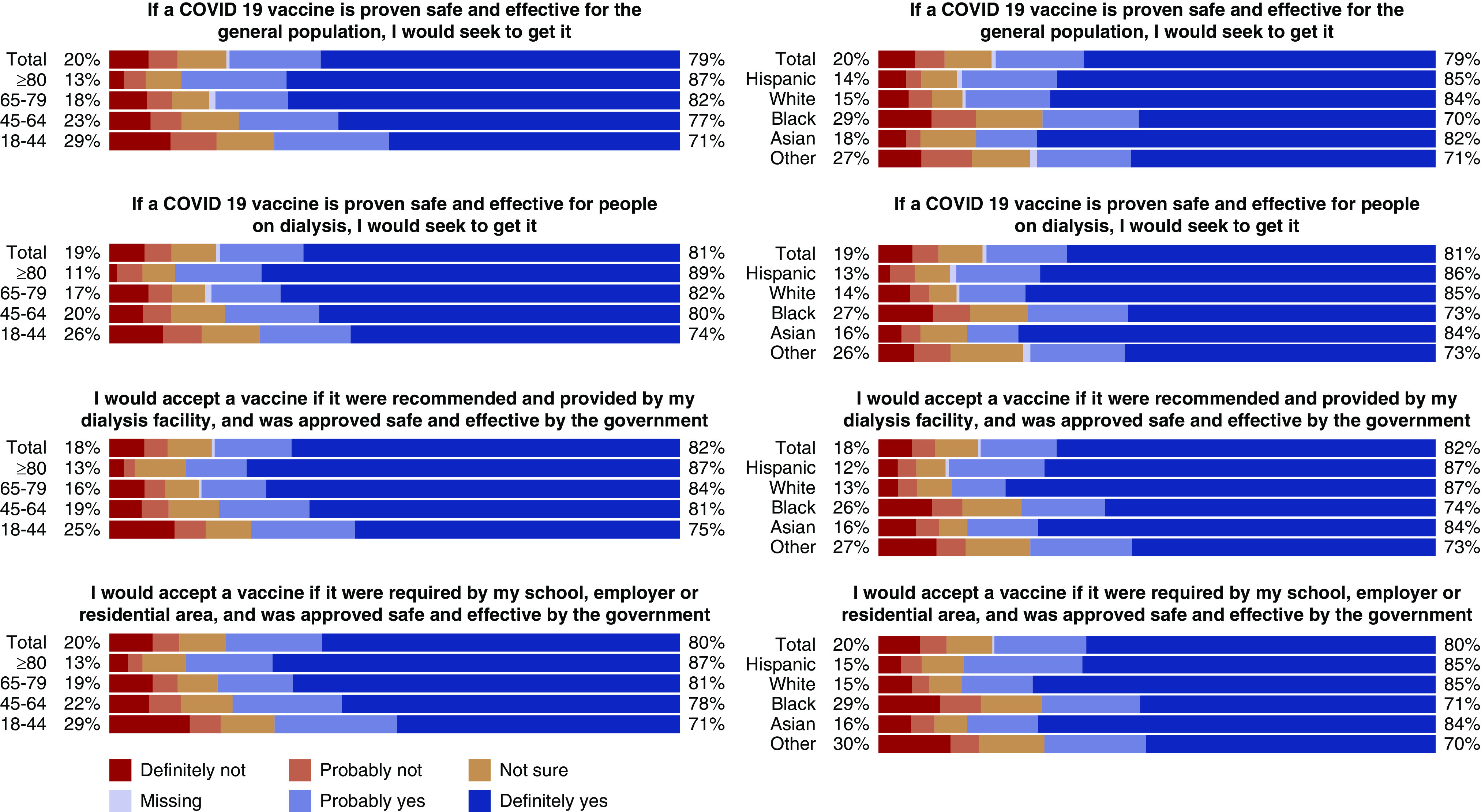
Responses to four vaccine acceptability questions by age and race/ethnicity. Responders in the age group 18–44 years had the lowest likelihood of vaccine acceptancy. Overall rates of vaccine hesitancy improved if vaccine was offered at dialysis facilities. Rates of definitive “no” were low ranging from 6% to 7.5% in the four vaccine acceptability questions.
Correlates of vaccine hesitancy included age, sex, race and ethnicity, level of education, death of a family member from COVID-19, and whether the patient had received or was planning to receive the influenza vaccine. The odds of vaccine hesitancy were higher among patients aged 18–44 years (OR, 1.5; 95% CI, 1.0 to 2.3), women (OR, 1.6; 95% CI, 1.2 to 2.0), Black patients (OR, 1.9; 95% CI, 1.3 to 2.7), and patients identifying as “other” race or ethnicity (OR, 2.0; 95% CI, 1.1 to 3.7). The last category was comprised largely of patients identifying as Native Americans or Pacific Islanders. The odds of vaccine hesitancy were lower among patients aged ≥80 years (OR, 0.4; 95% CI, 0.3 to 0.7), among those with some college education (OR, 0.4; 95% CI, 0.2 to 0.6), and among those who received or were planning to receive the influenza vaccine (OR, 0.2; 95% CI, 0.1 to 0.3) (Figure 2, Supplemental Table 2). The overriding concern of patients who were vaccine hesitant related to side effects, followed by doubts on the efficacy of the vaccine, and being uncomfortable with vaccines in general (Figure 3). The main sources of information about the COVID-19 vaccines were television news (68%), followed by dialysis staff (38%). Younger responders were more likely to use social media as a source of information than older responders (Supplemental Figure 2).
Figure 2.
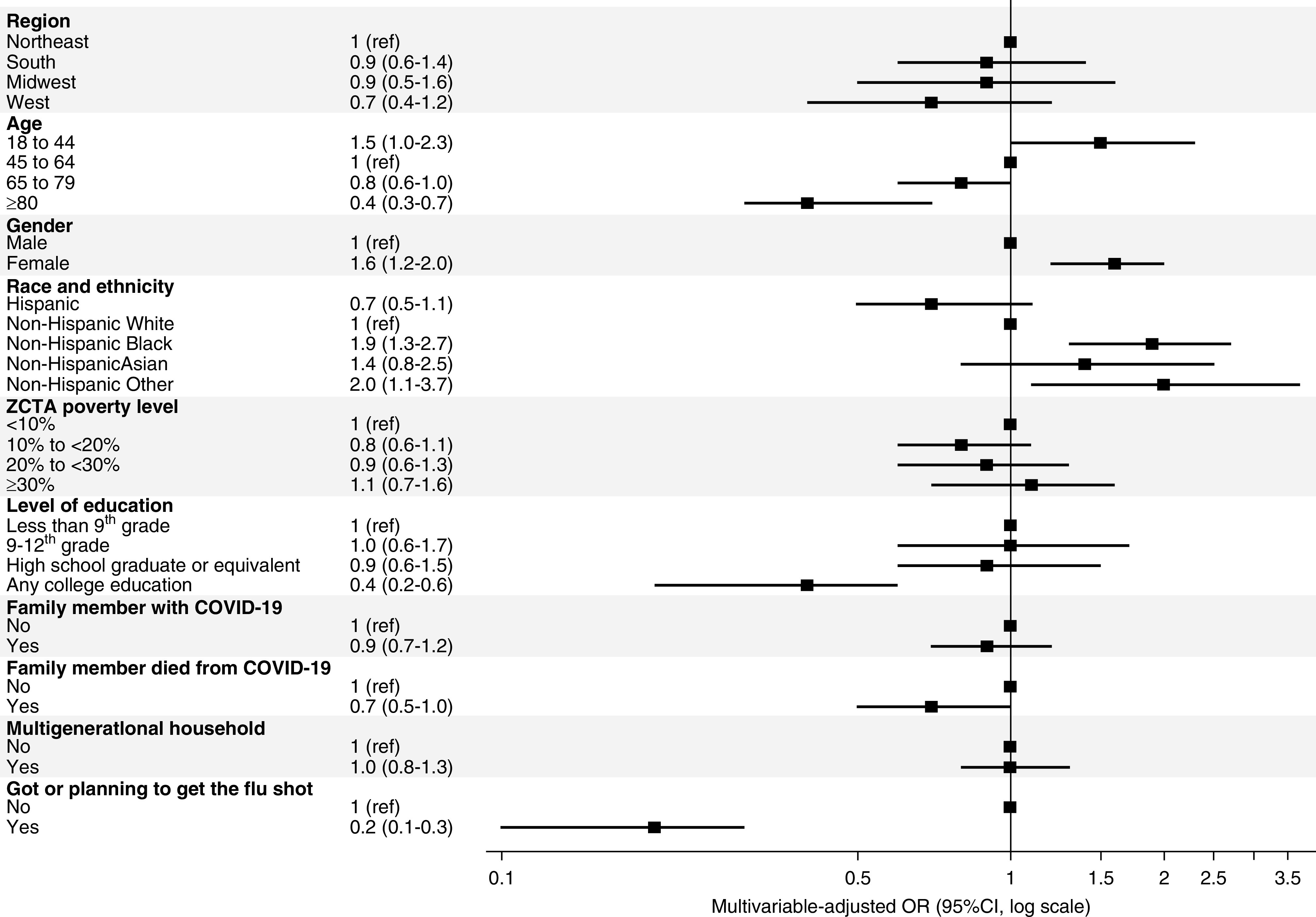
Correlates of vaccine hesitancy. The likelihood of vaccine hesitancy on one of four questions was lower among older, non-Black, and college-educated responders. Men, responders living in the West, responders in whose family or close circle someone had died from COVID-19, and responders who had received an influenza vaccine also had lower levels of vaccine hesitancy.
Figure 3.
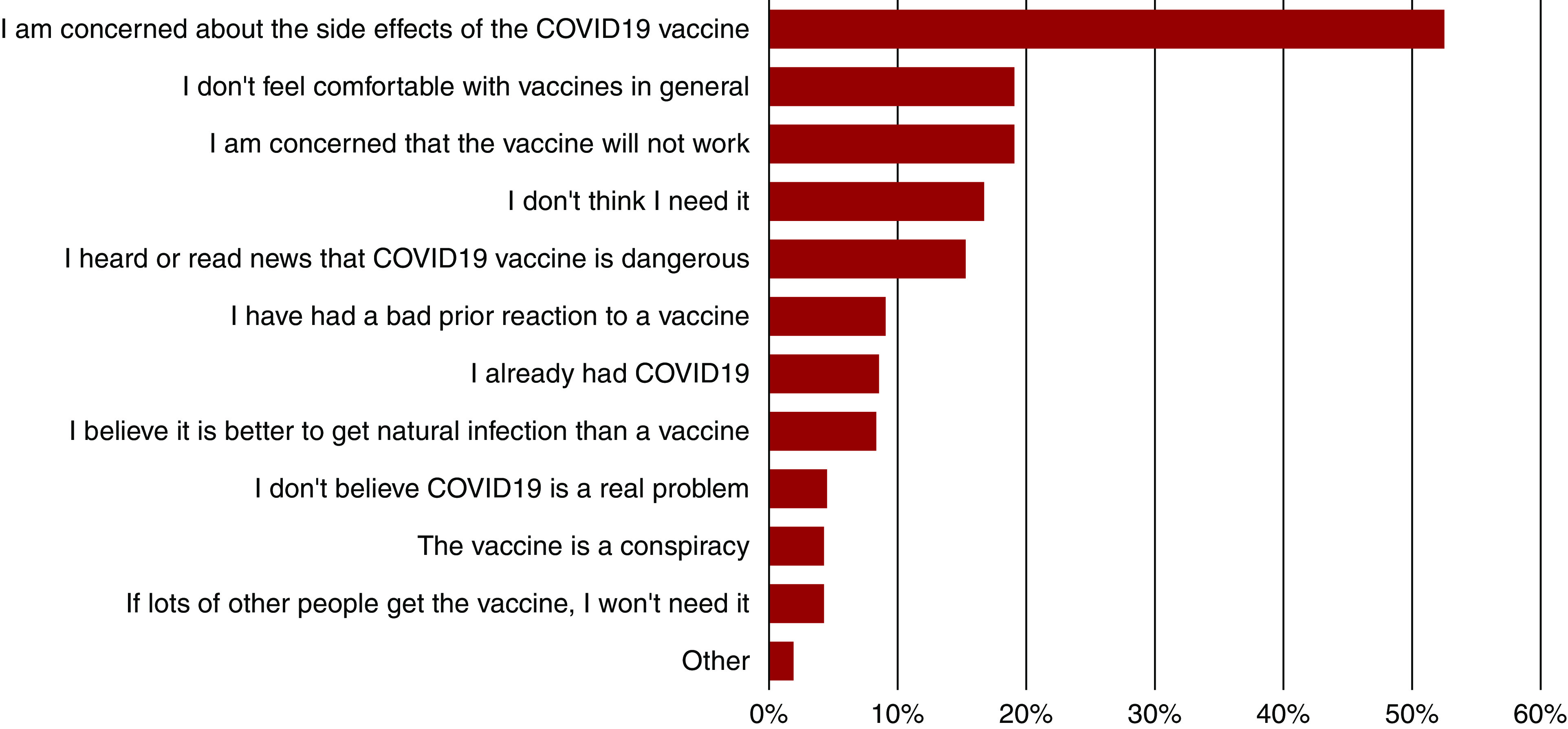
Reasons for vaccine hesitancy. In total, 53% of participants who were hesitant to receive the COVID-19 vaccine were concerned about its side effects; 12% believed it was dangerous; 19% were concerned about its efficacy, but a sizeable fraction was influenced by their general beliefs (19%) about or prior reaction to (9%) vaccines.
In summary, in our vaccine acceptability survey broadly representative of the US dialysis population, we found that approximately one in five patients on in-center hemodialysis was reluctant to receive the COVID-19 vaccine. Overall vaccine hesitancy rates were nearly half that of the most recently reported rates for the general US population.5,11 Characteristics of patients who were vaccine hesitant, however, were similar to those described for the general population4,5,7,12 and for the dialysis6 population: younger age, Black race, and lack of a college education. In contrast to results from the general population, however, we found that women receiving hemodialysis had a higher rate of vaccine hesitancy than men.
Surveys have described a change in vaccine acceptance over time in the United States, with one reporting an increasing acceptance,5 whereas a serial survey of the same population reported increasing hesitancy.11 Both report overall rates of vaccine hesitancy around 40% in early December, despite the release of data that two tested vaccines were likely to be highly efficacious. Higher rates of vaccine acceptance in patients on hemodialysis may indicate a greater level of trust or comfort with medical care in general, or an awareness of the population’s vulnerability to COVID-19. The higher rates of COVID-19 vaccine acceptance in our surveyed population compared with the general population are also compatible with 85% of our responders receiving or planning influenza vaccination, a proportion similar to available data on influenza vaccine coverage in the Dialysis Facility Report.17 Routine provision of influenza and hepatitis B vaccine in hemodialysis facilities may have encouraged higher vaccination acceptance overall.
The strengths of our survey include its broad and timely reach, including to Hispanic populations via the use of a Spanish language version and broadly representative sample.
The study’s limitations are the requirement of the interface with an online platform, which may be more challenging for older patients, patients with lower levels of education, and those with visual impairment.18 The first two categories were nonetheless well represented in our responders. Our response rate, although similar to another vaccine acceptability survey,4 was relatively low, possibly implying that a subpopulation with higher levels of health and technology literacy may have more likely engaged as responders. Some vaccine hesitancy questions that were adapted from a widely distributed international survey10 embedded complex concepts. In our pragmatic approach, responders did not have opportunities to ask for clarification. Another limitation is the absence of responses from the Northwestern region, and that the survey is a single time-point snapshot—vaccine acceptance may increase or decrease as data on efficacy and safety accrue over time.
Our results highlight opportunities for improving SARS-CoV-2 vaccine uptake through dialysis facilities. On the basis of these survey results, we would advise dialysis organizations and patient advocacy groups to focus vaccine promotion efforts among younger age groups, women, and Black, Native American, and Pacific Islander patients, and to develop patient-friendly educational material describing the rates and nature of vaccine-related adverse effects. The caveats that side effects and efficacy have not been specifically evaluated in patients on dialysis complicate outreach efforts. Dialysis care providers and public health agencies should capture data on safety (adverse effects) and provisional efficacy (seroconversion) in the dialysis population, and rapidly disseminate these data to facilitate vaccine uptake.
Disclosures
G. Block reports having consultancy agreements with Akebia, Keryx, Kirin, and Reata; has an ownership interest in Ardelyx and Reata; reports receiving research funding from Akebia, Ardelyx, and GlaxoSmithKline; reports receiving honoraria from Amgen and Kirin; reports being a scientific advisor or member of Ardelyx, CJASN, Kirin, and Reata; and other interests/relationships as former member of the Executive Summary Committee of Kidney Disease Improving Global Outcomes, former Medical Director at Davita, and previous employment with Reata. G.M. Chertow is on the Board of Satellite Healthcare, a not-for-profit dialysis organization; reports consultancy agreements from Akebia, Amgen, Ardelyx, AstraZeneca, Baxter, Cricket, DiaMedica, Gilead, Miromatrix, Reata, Sanifit, Unicycive, and Vertex; reports having an ownership interest in Ardelyx, CloudCath, Durect, DxNow, Eliaz Therapeutics, Outset, Physiowave, and PuraCath; reports receiving research funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Institute of Allergy and Infectious Diseases; reports being a scientific advisor or member as Co-Editor of Brenner & Rector’s The Kidney (Elsevier); and other interests/relationships with Data and Safety Monitoring Board service: NIDDK, Angion, Bayer, ReCor. J. Flotte reports being a scientific advisor or member of US Renal Care. S. Anand reports receiving research funding from Applied Pragmatic Research Grant from Satellite healthcare; reports receiving honoraria from American Kidney Fund; and reports being a scientific advisor or member of the International Society of Nephrology i3C and Consortium for the Epidemic of Nephropathy in Central America and Mexico. M. Montez-Rath reports receiving research funding from Sanofi. C. Fults is employed by and has ownership interest in US Renal Care. M.S. Block is employed by US Renal Care. M. Dittrich is employed by and has ownership interest in US Renal Care. M. Dittrich also has Ownership Interest in Signify Health, and Multiple dialysis units. All remaining authors have nothing to disclose.
Funding
P. Garcia was funded by the American Kidney Fund Clinical Scientist in Nephrology Award and the Stanford University School of Medicine Leeds Compassionate Scholar Award. G.M. Chertow was supported by NIDDK grant K24 DK085466. S. Anand was supported by NIDDK grant R01 DK127138.
Supplementary Material
Acknowledgments
The authors thank the participating dialysis facility governance team and staff for facilitating the survey.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021010104/-/DCSupplemental.
Supplemental Table 1. Distributions of age, sex, race/ethnicity and region in the survey responders, overall US Renal Care population in comparison to the US adult dialysis population obtained through the United States Renal Data System (USRDS).
Supplemental Table 2. Characteristics of survey responders by vaccine-hesitant status.
Supplemental Figure 1. Location of zip codes from which surveys were returned and sampled facilities.
Supplemental Figure 2. COVID-19 vaccine information sources.
Supplemental Survey. Surveys in English and Spanish.
References
- 1.Centers for Medicare and Medicaid Services : Preliminary Medicare COVID-19 data snapshot. 2021. Available at: https://www.cms.gov/research-statistics-data-systems/preliminary-medicare-covid-19-data-snapshot. Accessed February 20, 2021
- 2.Ng JH, Hirsch JS, Wanchoo R, Sachdeva M, Sakhiya V, Hong S, et al.; Northwell COVID-19 Research Consortium and the Northwell Nephrology COVID-19 Research Consortium: Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int 98: 1530–1539, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu CK, Ghai S, Waikar SS, Weiner DE: COVID-19 infection risk among hemodialysis patients in long-term care facilities. Kidney Med 2: 810–811, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM: Attitudes toward a potential SARS-CoV-2 vaccine: A survey of U.S. adults. Ann Intern Med 173: 964–973, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funk C, Tyson A: Intent to get a COVID-19 vaccine rises to 60% as confidence in research and development process increases, 2020. Available at: https://www.pewresearch.org/science/2020/12/03/intent-to-get-a-covid-19-vaccine-rises-to-60-as-confidence-in-research-and-development-process-increases/. Accessed January 21, 2021
- 6.Danziger J, Weinhandl E, Friedman D, Mukamal KJ: Racial and ethnic disparities in seasonal influenza vaccination among dialysis facilities in the United States. J Am Soc Nephrol 31: 2117–2121, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Foundation for Infectious Disease: National survey: black adult perspectives on COVID-19 and flu vaccines, 2021. Available at: https://www.nfid.org/national-survey-black-adult-perspectives-on-covid-19-and-flu-vaccines/. Accessed February 6, 2021
- 8.Rossi PH, Wright JD, Anderson AB: Handbook of Survey Research, Cambridge, Massachusetts, Academic Press, 2013 [Google Scholar]
- 9.Larson HJ, Jarrett C, Schulz WS, Chaudhuri M, Zhou Y, Dube E, et al.; SAGE Working Group on Vaccine Hesitancy: Measuring vaccine hesitancy: The development of a survey tool. Vaccine 33: 4165–4175, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabi K, et al.: A global survey of potential acceptance of a COVID-19 vaccine [published correction appears in Nat Med 27: 354, 2021 10.1038/s41591-020-01226-0]. Nat Med 27: 225–228, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szilagyi PG, Thomas K, Shah MD, Vizueta N, Cui Y, Vangala S, et al.: National trends in the US public’s likelihood of getting a COVID-19 vaccine-April 1 to December 8, 2020. JAMA 325: 396–398, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamel L, Kirzinger A, Muñana C, Brodie M: KFF COVID-19 vaccine monitor: December 2020, 2020. Available at: https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/. Accessed December 15, 2020
- 13.Rumbaut RG, Massey DS: Immigration and language diversity in the United States. Daedalus 142: 141–154, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Buuren S, Boshuizen HC, Knook DL: Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 18: 681–694, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Van Buuren S, Brand JPL, Groothuis-Oudshoorn CGM, Rubin DB: Fully conditional specification in multivariate imputation. J Stat Comput Simul 76: 1049–1064, 2006 [Google Scholar]
- 16.Little RJ, Rubin DB: Statistical Analysis with Missing Data, Vol. 793, Hoboken, New Jersey, John Wiley & Sons, 2019 [Google Scholar]
- 17.Centers for Medicare and Medicaid Services : Dialysis facility report, 2020. Available at: https://data.cms.gov/dialysis-facility-reports. Accessed February 20, 2021
- 18.Hussein WF, Bennett PN, Pace S, Chen S, Legg V, Atwal J, et al.: The mobile health readiness of people receiving in-center hemodialysis and home dialysis. Clin J Am Soc Nephrol 16: 98–106, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.